58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND351962-ND3: Synthesis and Characterization of Dyads Capable of Achieving Photoexcited Two-Electron Charge-Separated States
Raphael G. Raptis, PhD, Florida International University
The work of two graduate students has focused initially on the synthesis of complexes containing photosensitive electron donors connected to a multi-electron acceptor moiety. The multi-electron acceptor is in all three cases the octanuclear ferric complex [Fe8(m4-O)4(m-4-R-pz)12L4] (R = H and Me; pz = pyrazolato anion), which has been previously shown to accept up to four electrons in consecutive one-electron reduction processes at modest redox potentials. The parent L = Cl complex was used as starting material, as the chloride ligands are easily substituted in metathetical reactions, allowing the attachment of the photosensitive groups. Phenolato ligands are the preferred groups to introduce in the place of chlorides, as the resulting Fe-O bonds are quite inert, even in humid solvents. Therefore, the chosen synthetic strategy was to either use photosensitive aromatic alcohols, or functionalize other photosensitive groups (e.g., metalloporphyrins, or [M(bipy)3] complexes) with phenolic groups.
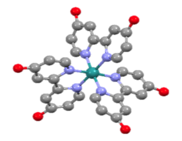
1. Synthesis of [Fe8(m4-O)4(m-4-R-pz)12(OAr)4], ArOH = 1-naphthol, 2-naphthol, or 6-substituted naphthols; five new compounds in total. The new compounds have been characterized by elemental analysis, 1H-nmr and X-ray crystrallography (e.g., Figure 1) and voltammetric methods. The assignment of the paramagnetically-shifted 1H-nmr resonances of all-five new compounds, containing information on the distribution of spin density over the phenolate ligands, while not directly related to the goals of this project was of particular interest: a linear correlation between chemical shift and redox potentials (for the first reduction process) was revealed. To the best of our knowledge, this relationship has not been described in the literature, to date.
Figure 1. Ball-and-stick diagram of the X-ray structure of [Fe8(m4-O)4(m-4-Me-pz)12(1-naphthol)4]; Fe, yellow; O, red; N, blue. Hydrogen atoms not shown.
2. Synthesis of Fe8/Fe-TPP conjugates. The complex [Fe8(m4-O)4(m-4-H-pz)12(OAr)4], Ar = 3-C5H3N (3-hydroxypyridine) was synthesized and characterized by elemental analysis, 1H-nmr and X-ray crystrallography. This complex contains four pyridyl functions available for further coordination. Addition of FeII-TPP (TPP = tetraphenylporphyrin) to this complex gives an Fe8/Fe-TPP conjugate with Fe8 to Fe-TPP ratio of 1-4, depending on reaction stoichiometry. Efforts to characterize the conjugates by X-ray crystallography have been, so far, unsuccessful. However, the coordination of dangling pyridine groups to the axial Fe-sites of Fe-TPP is evident by the gradual broadening and eventually flattening out of pyridine 1H-nmr resonances as the Fe8/Fe-TPP ratio increases from 1 to 4. The UV-vis spectra of the conjugates contain features of both the Fe8 and Fe-TPP components and are not informative. However, the emission spectra show a new band at 700 nm, which is not present in the emission spectrum of either Fe8 or Fe-TPP component.
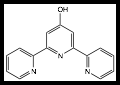
3. Synthesis of Fe8/[M(bipy)3]2+ and Fe8/[M(terpy)2]2+ conjugates. 4,4'-dihydroxybipyridine (DHBP) was synthesized by a literature method and the corresponding [M(DHBP)3]2+ complexes, M = Cu, Zn, Ru and Co, were prepared from MCl2 salts. The four compounds were characterized by elemental analysis and those of M = Ru and Co (not previously reported) also by X-ray crystallography (Figure 2). Efforts to coordinate the [M(DHBP)3]2+ complexes to Fe8 via the peripheral phenol groups have resulted in mixtures of products, which have not yielded single crystals appropriate for X-ray study yet.
Figure 2. Ball-and-stick diagram of the X-ray structure of [M(DHBP)3]2+, M = Ru, Co; M, green; O, red; N, blue. Hydrogen atoms not shown.
An alternative strategy has been followed towards the synthesis of Fe8/[M(terpy)2]2+ conjugates: hydroxylated terpy (hterpy, Scheme 1) has been prepared by a literature method. The corresponding [Fe8(m4-O)4(m-4-H-pz)12(hterp)4] complex has been prepared by substitution of the chloride ligands of the starting Fe8 material. The new complex has been characterized by elemental analysis, 1H-nmr and UV-Vis spectroscopy. Its UV-Vis spectrum shows the diagnostic band at ~600 nm, characteristic of phenol-group coordination to Fe8 complexes. This complex will be used to assemble new materials containing M(hterpy)2-functionalities, where the two hterpy-groups will be coordinated to two [Fe8(m4-O)4(m-4-H-pz)12(hterp)4] units.
Scheme1.
Preliminary photophysical studies of [Fe8(m4-O)4(m-4-R-pz)12(naphthol)4] complexes have shown efficient quenching of the naphthol emission upon coordination to Fe8. The relaxation of the photoexcited state takes place by two parallel mechanisms with half-lifes t1/2 of 2 and 5 ns, respectively, consistent with efficient energy transfer to the metal complex. This has discouraged further photophysical studies of the naphthol complexes. However, detailed photophysical studies of the Fe8/Fe-TPP, Fe8/[M(bipy)3]2+ and Fe8/[M(terpy)2]2+ conjugates are in progress.
Copyright © 2014 American Chemical Society