58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI751042-UNI7: Fundamental Studies of Swelling Hysteresis in Humidity Sensitive Polymer Films
Adam J. Nolte, PhD, Rose-Hulman Institute of Technology
The overarching goal of this work was to fabricate and investigate humidity swelling hysteresis in two thin polymer film systems. Humidity swelling hysteresis is the phenomenon by which a water-sensitive polymer film appears to display two different swelling ratios at the same relative humidity—the amount of swelling is dependent upon whether the humidity is increasing or decreasing. The origin of hysteretic swelling appears to lie in specific secondary interactions that are present within the polymer matrix, as well as the degree of polymer mobility, which can be controlled by factors such as crosslinking and solvent quality. Two polymer film systems were investigated in order to obtain fundamental insights into the types of interactions that cause hysteretic swelling in polymer films.
The first system comprised layer-by-layer (LbL) assemblies of poly(allylamine hydrochloride) (PAH) and poly(acrylic acid) (PAA). PAH/PAA LbL films are pH-sensitive, in that the crosslinking density of the films has been shown to be dependent upon the pH of the PAH and PAA solutions during assembly. The second system comprised thin films containing poly(N-isopropylacrylamide) (PNIPAAm). PNIPAAm films are temperature-sensitive, in that the temperature of the film dictates the solvent quality of water via an LCST phenomenon (the LCST is approximately 34 °C); at lower temperatures water is a better solvent for PNIPAAm. During Summer 2013 two undergraduate chemical engineering students conducted research under the direction of the PI on the above systems; their respective results are briefly summarized below:
Research Activity 1: “LbL films with controlled crosslink density and secondary interactions” (student: Ziyang Yin)
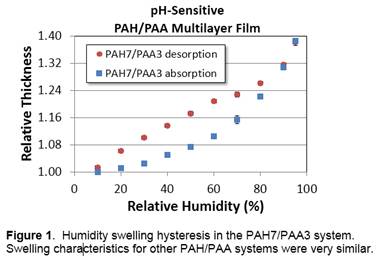
The goal here was to assemble PAH/PAA films at different pH combinations in order to examine how changes in electrostatic crosslinking may affect the humidity swelling hysteresis of the system. Other researchers had noted (Shiratori, Macromol 33, 4213), that the incremental per-bilayer growth increment of PAH/PAA can change drastically with pH, although this work was done for dip-coated films. We utilized spin-assisted assembly (SA-LbL), where the polymer solutions are directly spin-cast onto the substrate. We tested a combination of solution pH values ranging from PAH7/PAA3 (at this combination, films should be rather thick as PAA is only partially charged) to PAH7/PAA7 (both polymers are highly charged, so films should be rather thin). In addition, PAH7/PAA3 should correspond to less crosslinking than PAH7/PAA7. In all, five systems were tested; in each, the pH of PAH remained constant at pH 7, but the pH of PAA varied in unit increments from 3 to 7.
While SA-LbL yielded films with slightly different per-bilayer growth increments, the differences between systems were not near as significant as those that have been observed for dip-coated systems. We observed maximum and minimum per-bilayer growth increments for the PAH/PAA system of 9.9 nm/bilayer (for PAH7/PAA3 films) and 7.4 nm/bilayer (for PAH7/PAA7 films). This compares to approximately 12 nm/bilayer and < 1 nm/bilayer observed previously for PAH7/PAA3 and PAH7/PAA7 dip-coated films, respectively.
The similarity of our incremental growth data led us to hypothesize that the films should display similar maximum swelling ratios (at near 100% relative humidity (RH)) and that they should exhibit similar hysteresis trends. This indeed was found to be the case. The swelling curves for all systems were quite similar; for reference, one of these (for the PAH7/PAA3 system) is shown in Figure 1.
Future work will focus on better understanding the difference in growth increment between SA-LbL films and those constructed via dip assembly. In addition, dip-assembled films will be constructed to compare their swelling characteristics with their SA-LbL counterparts.
Research Activity 2: “Spin-coated, photo-crosslinked temperature sensitive polymer films” (student: Zhengyuan Fang)
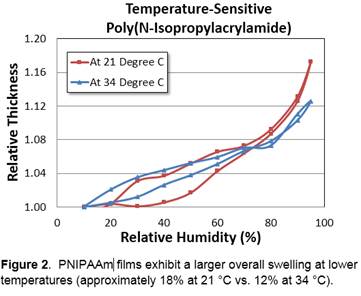
The goal of this part of the work was to obtain thin films of the temperature-responsive polymer PNIPAAm in order to conduct hysteretic swelling measurements as a function of temperature. At higher temperatures, where the polymer is known to be more hydrophobic, it was hypothesized that the overall swelling of the film would be less—we aimed to track trends in hysteresis of the films as well.
The primary goal for the first year on Research Activity 2 was to obtain a method for reliably fabricating crosslinked thin films of PNIPAAm. PNIPAAm can be obtained via polymerization of N-isopropylacrylamide, but only while restricting oxygen availability to the monomer (e.g., in closed spaces)—this can make very thin films (1 micron or less) difficult to fabricate. We were successful in incorporating PNIPAAm into polymer films by two different routes. In the first, PNIPAAm was incorporated via hydrogen-bonded LbL assembly with alginic acid. In the second, which turned out to be the route we settled upon, N-isopropylacrylamide monomer was sandwiched in between a hydrophobic silane-modified glass coverslip and a vinyl silane-modified silicon surface. It was compressed to create a confined liquid film of approximately 1 micron thickness, and then polymerized via UV light. The thickness of the resulting crosslinked PNIPAAm film could then be measured as a function of humidity in a temperature- and humidity-controlled chamber. Figure 2 shows preliminary experimental results from this work confirming the hypothesized lower overall swelling for a PNIPAAm film tested at higher temperature. Future work in the second year of the grant will focus on better understanding the LCST of PNIPAAm films in humid, as opposed to fully hydrated, environments. Swelling hysteresis of such films will also be closely investigated in order to ascertain the effects of the changing hydrophobicity of the films on the swelling behavior.
Overall, this first year of work allowed us to gain numerous important insights into thin film fabrication, and it revealed some surprises in terms of the similarity of SA-LbL film properties at different assembly pH. In the second year, we expect to dig deeper and utilize these insights into film fabrication to better understand hysteretic swelling phenomena in thin films.
For the PI, this research has provided an opportunity to expand my knowledge of film fabrication techniques for fundamental studies of swelling. For the participating students, this work has been providing opportunities to gain experience in the lab and expand their knowledge of polymer literature.
Copyright © 2014 American Chemical Society