58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI1052586-UNI10: Incorporation of Calix[4]crowns into Metal-Organic Frameworks for Hydrogen Storage
Xiangyang Lei, PhD, Lamar University
Our project goal is to develop new metal-organic frameworks (MOFs) with increased heat of hydrogen adsorption and enhanced hydrogen storage capacities at ambient temperature by incorporating bowl-shaped calix[4]crowns into organic linkers and their MOFs.
Progress
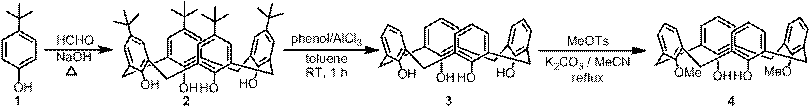
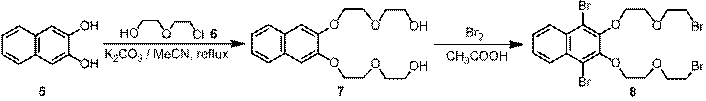
During the first ACS-PRF grant year, we focused on the synthesis and characterization of target organic linkers. Parent calix[4]arene (3) was chosen for the model study and the screen of reaction conditions for the synthesis of organic linkers. One new organic linker has been successfully synthesized (Scheme 1). As shown in Scheme 1, 1,3-dimethoxycalix[4]arene 4 and alkyl dibromide 8 are the two critical building blocks for the synthesis of target linker 12. Starting from p-tert-butylphenol (1), 1,3-dimethoxycalix[4]arene 4 was synthesized in three steps. The commercially available yet expensive p-tert-butylcalix[4]arene (2) was prepared in a large scale in good yield by the base-induced condensation of p-tert-butylphenol (1) with formaldehyde.1 The removal of the tert-butyl groups from the upper rim of p-tert-butylcalix[4]arene (2) led to calix[4]arene (3),2 which was converted to 1,3-dimethoxycalix[4]arene 4 by reacting with methyl tosylate in the presence of 1 equiv of K2CO3 in refluxing CH3CN.3 Meanwhile, alkyl dibromide 8 was prepared by the reaction of naphthalene-2,3-diol (5) with 2-(2-chloroethoxy)ethanol (6) to give diol 74 followed by bromination in acetic acid. The reaction of 1,3-dimethoxycalix[4]arene 4 and alkyl dibromide 8 yielded 1,3-bridged calix[4]crown 9.5 The Suzuki coupling reaction of calix[4]crown 9 with commercially available boronic acid 10 gave precursor 11, which was converted to linker 12 by basic hydrolysis followed by acidic workup. The structures of the intermediates and the target organic linker have been characterized by 1H and 13C NMR.
Scheme 1 Synthesis of a target organic linker (12)
We are working on the synthesis of more organic linkers with different linker length. Meanwhile, using linker 12 as the organic strut, we are investigating the reaction conditions for the synthesis of MOFs bearing calix[4]crown.
Impact
This ACS-PRF grant provided summer salary support for two undergraduate students, Billy Cao and Jordan King, during summer 2013. One graduate student, Anusha Alla, has been working on this project toward her M.S. degree. These students made great progress on this project and have gained valuable experience in literature search, organic synthesis, purification methods, instrumentation usage, and data analysis. The two undergraduate students were required to write a research report at the end of their research period. All of these students will present their research results at the annual poster competition in the Department of Chemistry and Biochemistry at Lamar University in October 2013. This practical, hands-on experience has helped these students develop creative and critical thinking skills that will facilitate their future studies and career.
References
5. (a) Gutsche, C. D., Calixarenes: An Introduction, 2nd Edition. The Royal Society of Chemistry: Cambridge, 2008; (b) Gutsche, C. D., Calixarenes Revisited. The Royal Society Chemistry: Cambridge, 1998; (c) Yamamoto, H.; Sakaki, T.; Shinkai, S., Regioselective Synthesis of 1,2- and 1,3- Bridged Calix[4]crowns. What are the Factors Controlling the Regioselectivity? Chem. Lett. 1994, 469-472.
Copyright © 2014 American Chemical Society