58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND751853-ND7: Synthesis and Design of New N-Heteroaromatic Materials
Tom G. Driver, University of Illinois (Chicago)
Interest in semiconducting polymers and fused-oligomeric aromatic molecules for charge transport and storage/conversion of energy has exploded because of their potential to be low-cost alternatives to inorganic materials, easy processibility, and tunable synthesis. For the past funding period, my group has pursued the synthesis of compounds that contain a thieno-[3,2-b]pyrrole structural motif because we anticipated them to exhibit promising and tunable electronic properties. Our syntheses were designed to leverage our C–H bond amination methods that our laboratory has developed. To achieve our goals, the following Specific Aims were proposed:
Specific Aim #1. Synthesize a range of polypyrrolothiophenes from monomers constructed via a Rh2(II)-catalyzed C–N bond formation and study the relationship between their molecular structure and bulk electronic properties.
Specific Aim #2. Synthesize novel fused oligo-N-heterocycles and compare their bulk properties to acenes.
Over the next few paragraphs, I detail our pursuit of these two Aims:
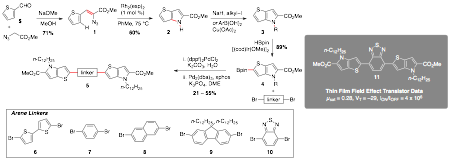
A series of new highly soluble bispyrrolothiophenes were synthesized by Crystalann Jones, a fifth year African-American graduate student in my group, from vinyl azides using transition metal-catalyzed C–H bond functionalization (Scheme 1). In addition to modifying the substituents present on the end-pyrrolothiophene moieties, the arene linker in between the two was also varied. The solution-state properties and field effect transistor (FET) electrical behavior of these bispyrrolothiophenes was compared and contrasted. Our investigations identified that the optical properties and oxidation potential of our compounds were dominated by the pyrrolothiophene unit with a λmax at ~400 nm and oxidation occurring at ~1 V. FET devices constructed with thin films of these bispyrrolothiophenes were also fabricated via thin-film solution processing. One of these compounds, a bispyrrolothiophene linked with benzothiodiazole exhibits a mobility of ~ 0.3 cm2/(V s) and an Ion/Ioff of >106.
Scheme 1. Synthesis and thin film field effect transistor properties of bispyrrolothiophenes.
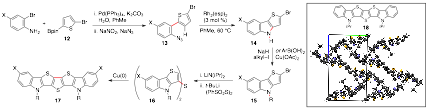
In addition to constructing low-molecular weight flexible oligomers, our group has also achieved the synthesis of N-heterocyclic heptacenes that contain a repeating fused-array of indoles and thiophenes (Scheme 2). Fei Zhou, a fourth year graduate student in my group, worked out the modular strategy to synthesize an array of these acenes and found that the reduction and oxidation potential could be tuned by the identity of the substituents on the arene end caps. While the identity of the N-substituent had little effect on the electronic properties of his compounds, Fei found that they exerted a strong effect on the solid-state crystal packing structure of his acenes. We will build on these promising results to determine the ideal N-substituent to reliably form a "bricklayer" crystal, which has been identified as the ideal isoform for electron-movement through an organic field effect transistor.
Scheme 2. Synthesis of N-heterocyclic acenes.
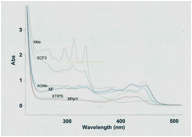
The third project that we pursued was to synthesize cruciform molecules using pyrrolothiophenes on the electron-donating axis (Scheme 3). While the other two projects were headed up by my graduate students, this project was executed by an undergraduate, Michelle Lee, who based her Honors College capstone project on it. Michelle used our Rh2(II)-carboxylate catalyzed C–H bond amination reaction to synthesize the thieno-[3,2-b]pyrrole pieces and then attached them to a central benzene ring through creation of the central benzobisoxazole. Then she attached a variety of acetylenes using a Sonogashira reaction. Michelle found that by changing the identity the acetylenic substituent, she was able to tune the UV/Visible spectrum of the cruciform solution.
Scheme 3. Synthesis and spectral properties of bispyrrolothiophene cruciforms.
In conclusion, we have made significant progress to synthesize a diverse array of novel N-heterocycles that exhibit a range of promising electronic properties. We will build on these results to design and synthesize new organic materials that exhibit better charge mobilities in transistors or function as fluorescent probes.
Copyright © 2014 American Chemical Society