58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND652618-ND6: Nonlinear Light Scattering from Micelles and Emulsion: Development of a Versatile Probe of Structure and Kinetics
Grazia Gonella, PhD, Temple University
Hai-Lung Dai, PhD, Temple University
Introduction. Notwithstanding the sprawling efforts in search of alternative energy sources, according to the latest International Energy Agency report, as of 2011, 31.5% of the world's energy was still being supplied by petroleum. The importance of surface chemistry and, specifically, colloids and surfactants in petroleum technologies has been widely acknowledged as micelles and emulsions play a ubiquitous role from enhanced oil recovery to environmental remediation. The aim of the present project is to develop nonlinear light scattering (NLS) techniques as a new tool for the study of the structure and kinetics of micelles and emulsions. In this first year, we have focused on the study of micellar systems.
Background. The term NLS includes, among others, hyper-Rayleigh scattering (HRS) and second harmonic generation (SHG). An isotropic solution of molecules with non-zero first order hyper-polarizability upon irradiation of light at frequency ω should not produce a response at frequency 2ω as the SH fields generated by the random distribution of molecules should cancel out. Nonetheless, a signal at frequency 2ω, known as HRS, generated by fluctuations in the orientation and density of the molecules in solution, can still be detected. Such signal is incoherent and scales linearly with the number of SH active molecules. However it has been proven that when these molecules are adsorbed on the surface of a nano/micro particle there is a specific relationship between the phase of the SH field they emit that results in a coherent response, called SHG. The coherence of SHG makes the signal quadratically proportional to the number of emitters. In the dipole approximation, when the bulk of the particle and the external medium present centrosymmetric structures, SHG arises solely from the interfacial region where inversion symmetry is broken.
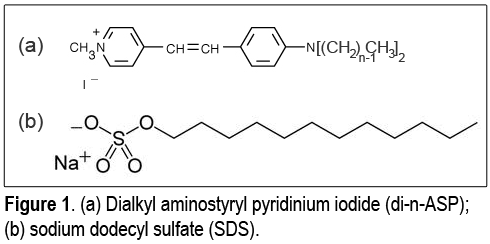
An important aspect of micelle formation is the concentration threshold, known as the critical micelle concentration (CMC), at which the surfactants self-aggregate. In order to exploit NLS as a probe of micelle formation we started looking for molecules that are both amphiphilic and SH active, absorbing either at ω or 2ω in order to obtain resonance-enhancement of the signal: Unfortunately, very few of the commercially available molecules fit these requirements. This finding pointed our work toward using these molecules as probes for formation of micelles by other surfactants instead of studying their own self-aggregation. Several probes were screened and finally the choice fell on the dialkyl aminostyryl pyridinium dyes (di-n-ASP) shown in Figure 1(a). Given the positive charge of the chromophore, di-n-ASP appears to be particularly well suited for the study of anionic amphiphiles such as the model surfactant sodium dodecyl sulfate (SDS), shown in Figure 1(b). Di-n-ASPs are available with different chain lengths: we chose n=4 as this chain is long enough to warrant inclusion of the molecule in the anionic micelle, because of the hydrophobic effect, but not too long to disrupt the SDS micellar structure. Moreover, di-4-ASP is water-soluble at concentrations below 30μM, which is important, because it can be used without introducing an additional phase (alcohol) in the solution.
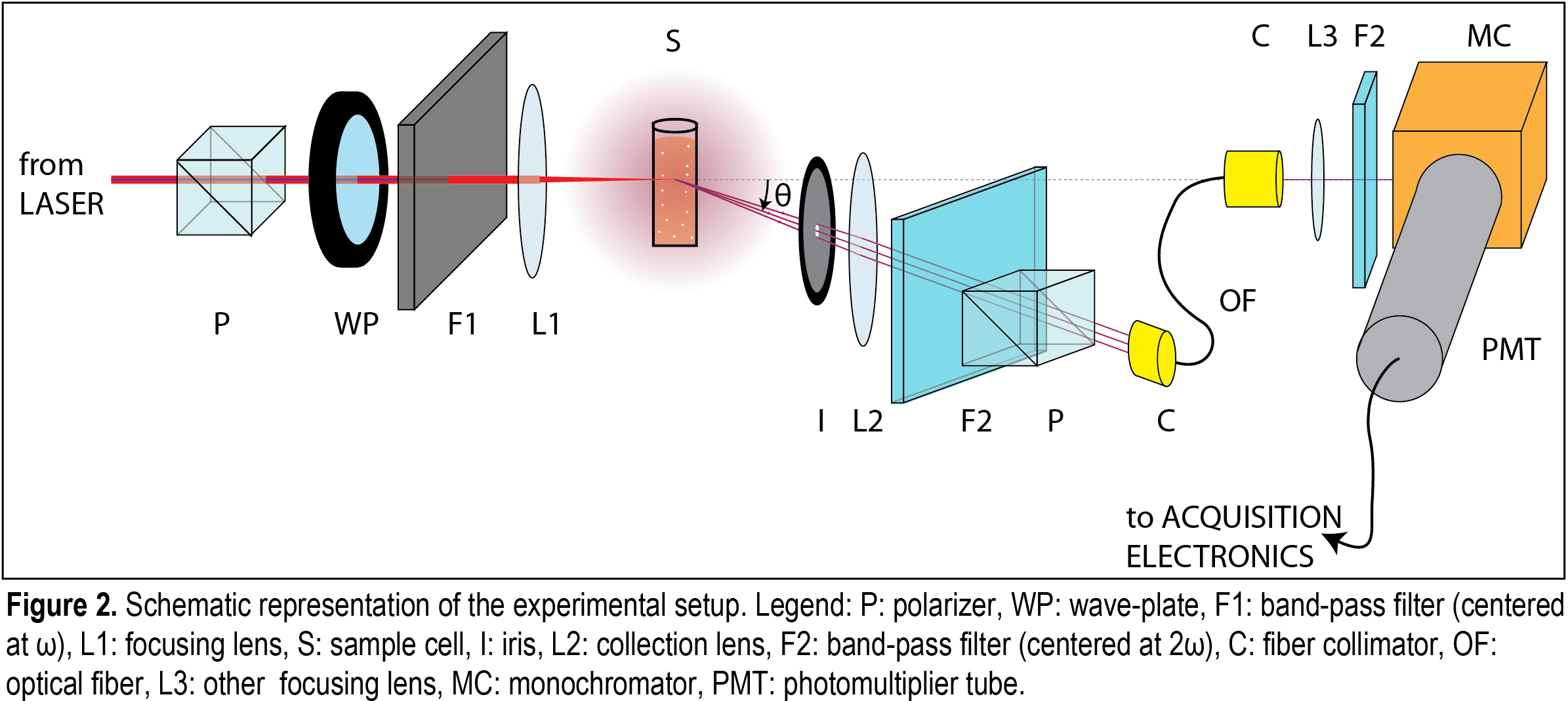
Experimental Methods. Our home-built NLS setup is shown in Figure 2. Briefly, the fundamental light from a femtosecond Ti:Sapphire laser (oscillator only, 800 nm fundamental wavelength, 80 MHz repetition rate, 100 fs nominal pulse duration, 400mW power) is focused down to ~50 μm into the liquid sample, which is held in a cylindrical glass cell. The scattered SH photons can be detected with angular resolution by using collection optics mounted on an arm rotating around the center of the cell. The acquisition wavelength is set by a monochromator. A photomultiplier tube is used for the detection of the SH signal that is amplified, processed by a single-photon counting system, and sent to a pc. Dynamic light scattering (Zetasizer Nano ZSP, Malvern) is routinely used to characterize our colloidal solutions.
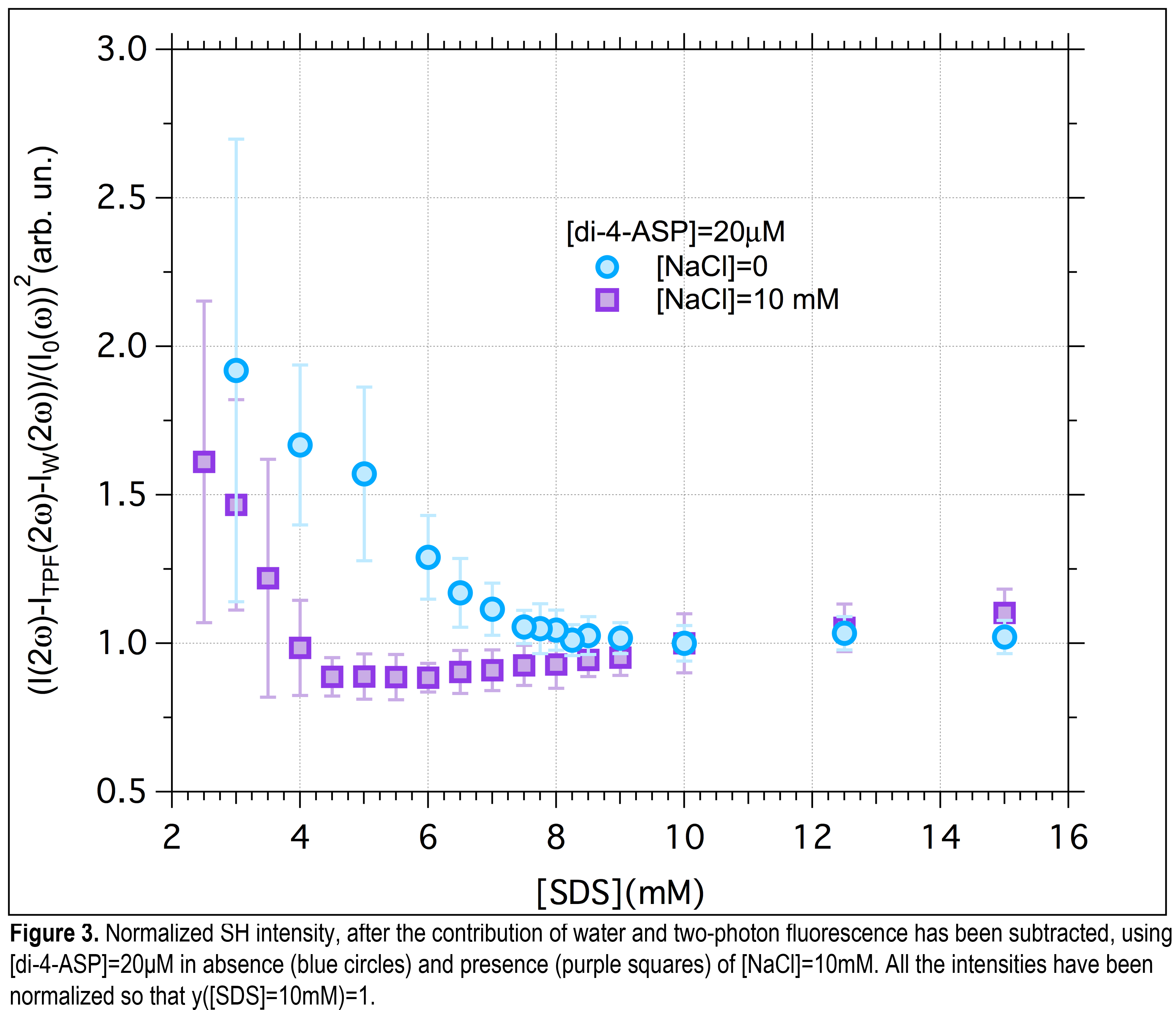
Results & Discussion. In this first phase, NLS dilution experiments using [di-4-ASP]=20μM have been carried out using s-polarized fundamental light and detection of SH intensity in the forward direction without polarization selection. At such low concentration, on average, there will be at best one di-4-ASP per micelle. The preponderant amount of s-polarized SH emission supports the fact that the signal is from HRS single di-4-ASPs, as the s-in/s-out polarization configuration is forbidden for SHG scattered from spherical objects. In Figure 3 the normalized SH signal from di-4-ASP is reported as a function of the [SDS] in aqueous solution. CMC of SDS at room temperature is known to be ~8.1mM and addition of a small amount of dye is not expected to change this value considerably. From the data in Figure 3 at [SDS]~ 8mM a change in the slope of the SH signal takes place: This behavior closely resembles that of other quantities such as osmotic pressure, turbidity and conductivity that show a slope discontinuity at the CMC when plotted against the surfactant concentration. To test our hypothesis, and study the effect of salt on CMC, NaCl has been added to the solution to reach [NaCl]=10mM. It is clear from Figure 3 that in presence of salt the slope change takes place at [SDS]~4.5mM which agrees well with the reported CMC value of ~5mM.
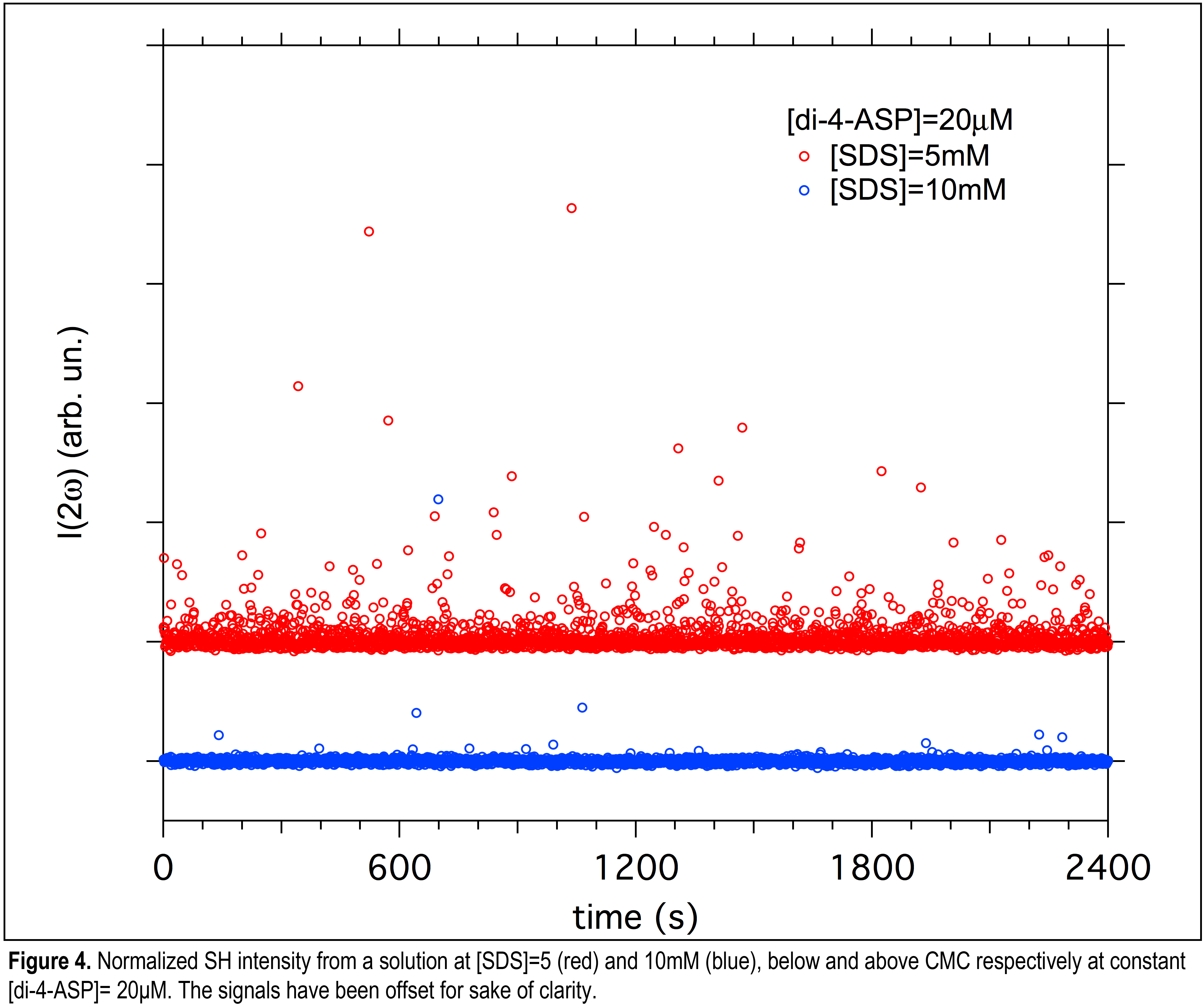
Through these studies another interesting observation has been made: SH signal fluctuations increase as [SDS] is decreased below CMC as shown in Figure 4.
Conclusions & Future Directions. In this first year, we have successfully applied the NLS method as a soft-matter probe and proven that NLS can be successfully used to determine CMC. The underlying cause will be further investigated in the coming months together with the observed fluctuations of the SH signal. As previously planned, the study can now be extended to include emulsions and even more complex systems. Because of this research, the students, the PI and Co-PI have been introduced to issues related to the formation and stability of micelles and emulsions. The application of the NLS will provide a new probe for characterizing these and more complex systems and contribute to solving some of the currently most challenging problems in colloid science.
Copyright © 2014 American Chemical Society