58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI352181-UNI3: Redox-Active Aluminum Complexes for Application in Carbonyl Reduction
Christopher R. Graves, PhD, Albright College
Progress in this first year of the grant has focused on the synthesis and characterization of aluminum complexes of redox active ligands. We have focused on two different ligands sets, the a-diimines and the 2-pyridyl hydroxylamines, both ligands that have been shown to exhibit rich redox chemistries. The following sections highlight the progress we have made in both projects.
Part 1 – Synthesis and Characterization of Aluminum-Diimine Complexes
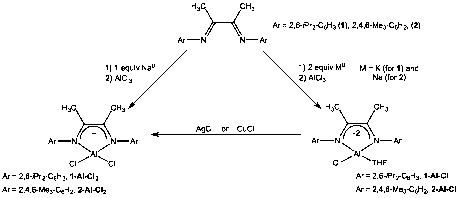
Diimine ligands were synthesized and characterized and their coordination chemistry to aluminum across several oxidation states was investigated (Figure 1). We specifically focused on the Ar-N=C(CH3)-C(CH3)=N-Ar ligands where Ar = 2,4-iPr2-C6H3 (LDiip, 1) and Ar = 2,4,6-Me3-C6H2 (LMes, 2). The doubly reduced (diimine-2)AlCl(THF) complexes 1-Al-Cl and 2-Al-Cl were prepared in good yield on a 1-gram scale via reduction of the ligand with 2 equiv of alkali metal followed by reaction with AlCl3. Reaction of the singly reduced ligands with AlCl3 affords the analogous (diimine-1)AlCl2 complexes 1-Al-Cl2 and 2-Al-Cl2, although we have found that controlling the ligand reduction chemistry is difficult and reaction products are contaminated with the doubly reduced complexes. The singly reduced complexes are more cleanly prepared by oxidative functionalization of the (diimine-2)AlCl(THF) complexes with either AgCl or CuCl. These results show that that the aluminum(III) ion can support both reduced states of the diimine ligand and that the redox properties of the complex can be tied to reaction chemistry at the aluminum ion.
Figure 1. Synthesis of aluminum complexes of the diimine ligand.
The Al-diimine complexes were characterized using various techniques, including multinuclear NMR spectroscopy, X-ray crystallography, Density Functional Theory, and electrochemistry. The solid-state molecular structures for 1-Al-Cl2 and 2-Al-Cl (Figure 2) corroborates that the ligand has been coordinated to the aluminum ion in the oxidation state proposed. The full geometries of the complexes were optimized using DFT and the theoretical bond distances and angles are in good agreement with those obtained in the X-ray analysis. The DFT calculations carried out on the Ar = iPr2-C6H3 system show that the SOMO of 1-Al-Cl2 and the HOMO of 1-Al-Cl are comprised primarily of a ligand based orbital with a bonding interaction between in the C-C backbone and an antibonding interaction in the C-N. This is in agreement with the structural data which show that the C-C bond length of the ligand backbone decreases systematically from free ligand to singly reduced complex to doubly reduced complex, while the C-N imine bonds get longer.
Figure 2. Solid-state structures of (LDiip1-)AlCl2 (1-AlCl2, left) and (LMes2-)AlCl(THF) (2-AlCl, right). Ellipsoids are at 30% and H atoms are omitted for clarity.
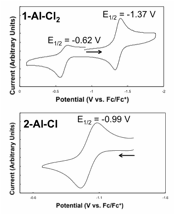
Electrochemical characterization of the complexes was also carried out, and the cyclic voltamagrams for the 1-Al-Cl2 and 2-Al-Cl complex are shown in Figure 3. The singly reduced 1-Al-Cl2 complex shows a reversible reduction process at ~-1.3 V, and a reversible oxidation process at -0.62 V. The doubly reduced 2-Al-Cl complex shows a singe reversible oxidation process at -0.99 V. These results demonstrate that aluminum supports the diimine ligand over multiple redox states and that the complexes exhibit reversible redox chemistry.
Figure 3. Cyclic voltammograms of compounds 1-Al-Cl2 (top) and 2-Al-Cl (bottom).
Part 2 – Synthesis and Characterization of Aluminum-Nitroxide Complexes
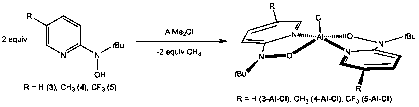
We also began to investigate the coordination chemistry of 2-pyridyl hydroxylamines to aluminum. Reaction of two equivalents of ligand precursor 3-5 with AlMe2Cl affords the [2-Npy,O-py-2-N(t-Bu)O]2AlCl 3-Al-Cl-5-Al-Cl in excellent yield (Figure 4). The reaction appears to be very general, and incorporation of electron donating (Me in 4) and withdrawing (CF3 in 5) groups are tolerated. The complexes were characterized by 1H and 13C NMR spectroscopies, which show all of the expected ligand resonances, and the solid-state molecular structures (Figure 5) confirm coordination of two equivalents of ligand to the aluminum ion. Our preliminary cyclic voltammetry experiments of complexes show reversible redox features, indicating the that the redox behavior of the ligand has been transfer to the aluminum complexes.
Figure 4. Synthesis of aluminum complexes of the 2-pyridyl hydroxylamine ligands.
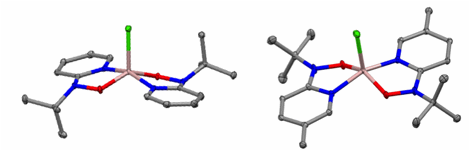
Figure 5. Solid-state structures of [2-Npy,O-py-2-N(t-Bu)O]2AlCl (3-Al-Cl, left) and [2-Npy,O-5-Me-py-2-N(t-Bu)O]2AlCl (4-Al-Cl, right). Ellipsoids are at 30% and H atoms are omitted for clarity..
Summary: In the first year of this project we have prepared and characterized two different classes of aluminum complexes implementing redox active ligands. Importantly, we have shown that the Al-complexes exhibit multielecron redox properties. These results will serve the basis for the next phase of the project, which will be investigate the reaction chemistry of the redox active aluminum complexes and ultimately to expand the reactivity profile of aluminum.
Four undergraduate research students have been supported by this PRF. This research opportunity has exposed the students to a diverse skill set, including the synthesis and handling of air-sensitive compounds and several characterization tools. This training will have a huge impact for future opportunities for the students, as they will be more competitive when applying for graduate school or for industrial positions.
We begun to disseminate the results of this project through presentations at regional and national meetings. Both the PI and the students working on the project have attended meetings, including posters at several local meetings, the 244th ACS National Meeting held in Philadelphia, and the 20th EuCheMS Conference on Organometallic Chemistry, held in St. Andrews, Scotland. We are also planning on submitting abstracts to the upcoming 247th ACS National Meeting being held in Dallas.
Copyright © 2014 American Chemical Society