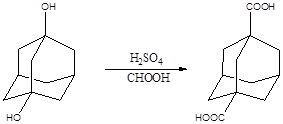58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI751833-UNI7: Epoxy Based Molecular Composites Containing Integrated Diamondoids
Luyi Sun, PhD, Texas State University
Diamondoids are a group of hydrocarbon cage molecules with unique physiochemical properties because of their diamond-like structure. They could be ideal fillers to modify polymers at the molecular level. The diamondoid derivatives can be considered as co-monomers, which can be well integrated into the polymer matrices via chemical crosslinking, leading to significant property improvement.
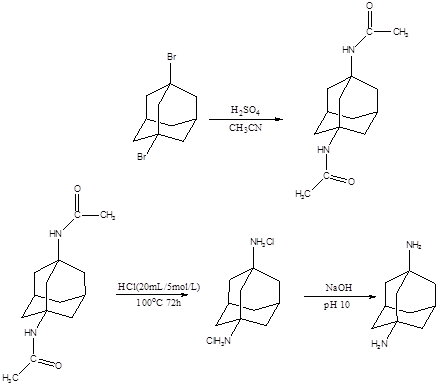
During the first year of this project, we synthesized two types of diamondoid derivatives, which can serve as fillers for the preparation of epoxy based molecular composites. 1,3-Adamantanedicarboxylic acid was obtained by the reaction of 1,3-adamantanediol (from Sigma-Aldrich) with H2SO4 (98%) in the presence of formic acid at room temperature (Scheme 1). After reaction optimization, we achieved ca. 94% yield of this reaction. Another adamantane derivative, 1,3-diaminoadamantane (Scheme 2), was obtained by the hydrolysis and neutralization of 1,3-diacetamidoadamantane, which was prepared by the reaction of 1,3-dibromoadamantane (from Sigma-Aldrich) with H2SO4 (98%). The final yield of 1,3-diaminoadamantane was ca. 60%. The structures of the above two adamantane derivatives were confirmed by nuclear magnetic resonance (NMR) and Fourier transform infrared spectroscopy (FT-IR) characterization. The thermal stability of these two adamantane derivatives were characterized by thermal gravimetric analysis (TGA), which showed that the adamantane derivatives could be thermally stable up to 200 oC. Therefore, they can sustain epoxy curing temperature and match the critical requirement as a molecular filler for epoxy.
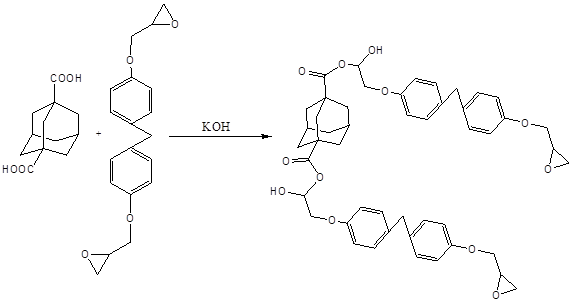
1,3-Adamantanedicarboxylic acid was further reacted with epoxy monomers (EPON862) using KOH as a catalyst (Scheme 3). The NMR and FT-IR characterization of the resultant product showed that the adamantane skeleton was successfully grafted to the EPON862 monomers after the reaction. The thermal stability of the epoxy monomers containing adamantane skeleton was significantly enhanced. The decomposition temperature of the modified epoxy monomers after the incorporation of 1,3-adamantanedicarboxylic acid was improved from ca. 250 to 350 oC. This result supports the hypothesis that the rigid cage structure of adamantane could effectively enhance the thermal stability of polymers.
Scheme 1
Scheme 2
Scheme 3
Both 1,3-diaminoadamantane and modified epoxy monomer grafted with 1,3-adamantanedicarboxylic acid were used to prepare epoxy/adamantane molecular composites as proposed, with the former used as a co-curing agent while the later used as a co-monomer. Samples of the above two molecular composites containing adamantane cages have been successfully prepared.
Due to the job change of the PI from a non-doctoral department to a Ph.D.-granting department, this grant was ended one year before the initially planned schedule. But the PI is continuing this project under the support of his start-up fund from the new institute. The PI expects to publish another 2 papers in the near future and the ACS PRF fund will be gratefully acknowledged.
Although this is a relatively small grant, such a seed fund had a significant impact on the PI. One year after receiving this ACS PRF grant, the PI secured three grants, one from Air Force Office of Scientific Research, one from the Norman Hackerman Advanced Research Program of the State of Texas, and one from the National Science Foundation. Two of the grants are highly related to the work initiated from this ACS PRF grant.
This ACS PRF grant also had a significant impact on the career of several students. Among the three students conducted the research under the support of this ACS PRF grant, two of them have moved for higher education. Jingfang Yu (was a Master student) is now a PhD student in the University of Connecticut, while Adam Oliphant (was an undergraduate student) is now a graduate student in Virginia Tech. Another undergraduate student who did not receive stipend directly from this grant but worked on this project, Jarett Martin (was an undergraduate student), is now a PhD student in Texas A&M University. The PI himself also benefited a lot from this grant. As stated above, the PI received three grants one year after this ACS PRF grant.
Copyright © 2014 American Chemical Society