58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR152162-UR1: Development and Application of Air-Stable, Shvo-Type Iron Catalysts
Timothy W. Funk, PhD, Gettysburg College
We are broadly interested in developing air-stable (cyclopentadienone)iron carbonyl compounds as catalysts for reductive and oxidative transformations of organic compounds. Our interest in these compounds stems from their ease of synthesis, their ease of handling, and the low cost (and high abundance) of iron. During our first grant period we focused our efforts largely on two aspects of the proposed research: exploring the reactivity of known (cyclopentadienone)iron carbonyl compounds as catalysts for the lactonization of diols, and synthesizing a series of (cyclopentadienone)iron tricarbonyl compounds with varying cyclopentadienone substitution and testing their reactivities in a series of oxidative and reductive transformations.
Diol Lactonization
Iron compounds 1 and 2 were previously synthesized by Knölker, and we discovered that under certain conditions they are both active alcohol oxidation and carbonyl reduction catalysts (Scheme 1). We view these compounds as air-stable variants of Knölker's air-sensitive iron hydride 3, which was shown to be an active transfer hydrogenation catalyst by Casey. Our initial studies of the reactivity of 1 and 2 suggested that they could catalyze the oxidative cyclization of diols to lactones.
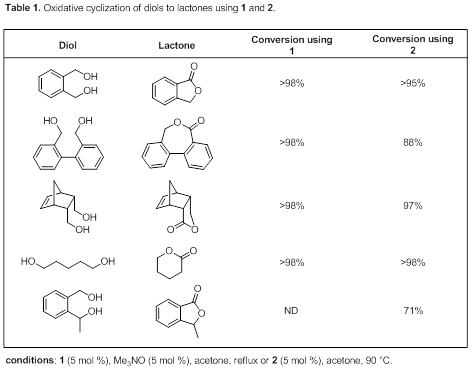
We began by exploring how 1 and 2 catalyzed the lactonization of symmetrical diols. Lactones containing 5, 6, and 7-membered rings could all be formed in high conversion using conditions similar to those used for simple alcohol oxidations. A few of the substrates tested are shown in Table 1. Catalyst 1 is activated by treating it with trimethylamine N-oxide to remove one CO ligand, and catalyst 2 must be heated to 90 °C for it to be an active catalyst. In most cases catalyst loadings of 5 mole % were used. Even linear diols like 1,5-pentanediol readily formed rings. Both catalysts appeared to have approximately equal activity when cyclizing symmetrical diols.
We have recently turned our attention to exploring the reactivities of unsymmetrical diols. In these substrates the primary alcohol must be selectively oxidized in the presence of a secondary alcohol. Our initial exploration of an unsymmetrical diol suggested that these catalysts are active in unsymmetrical diol oxidations (last entry of Table 1).
Currently we are synthesizing unsymmetrical diols (including enantioenriched diols) and we will test how they react with catalysts 1 and 2.
Substituted (Cyclopentadienone)Iron Tricarbonyl Synthesis and Activity
There have been a few studies of the catalytic activities of substituted (cyclopentadienone)iron tricarbonyl compounds as alcohol oxidation and carbonyl reduction catalysts. A vast majority of the iron compounds studied contained cyclopentadienones that were part of fused ring systems (such as those found in 1, 2, and 3). During our initial exploration of tricarbonyl compound 1 we examined the reactivity of a few derivatives of 1. We found that the cyclopentadienone substitution had a large impact on the catalyst's activity. One of the goals of our research was to synthesize a series of (cyclopentadienone)iron tricarbonyl compounds with systematic variations in the cyclopentadienone substitution and test these compounds for their catalytic activities. Our hope was to discover a structure/activity relationship and to use it to develop more active catalysts.
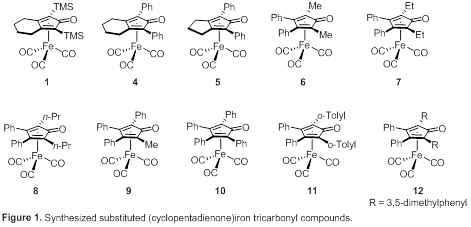
We began by synthesizing a series of known and novel (cyclopentadienone)iron tricarbonyl compounds, which are shown in Figure 1. Compounds 1, 4, and 5 have rings fused to the cyclopentadienone. Iron compounds 6–12 have phenyl groups in the 3 and 4 positions of the cyclopentadienone ring and have progressively bulkier groups in the 2 and 5 positions. All of the compounds in Figure 1 are stable in air.
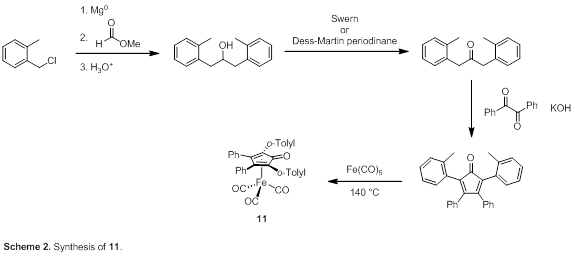
For compounds 1, 4–10, and 12 either the iron compound or the cyclopentadienone was known. The complexes are accessible by treating Fe(CO)5 with the appropriate cyclopentadienone at elevated temperature followed by purification by column chromatography. Our synthesis of the o-tolyl-containing cyclopentadienone and the corresponding catalyst (11) is shown in Scheme 2.
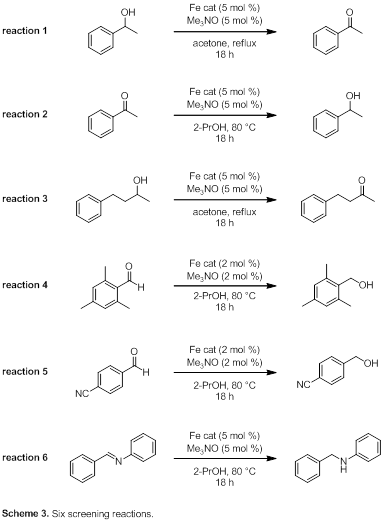
We currently have useful quantities of all of the catalysts in Figure 1, and we have begun to screen them for activity in the six oxidation and reduction reactions in Scheme 3. Reactions 1 and 2 are simple oxidation and reduction reactions, respectively. We have established that compound 1 is only modestly active in the oxidation of aliphatic alcohols, and reaction 3 tests how well aliphatic alcohols are oxidized by the other catalysts. The aldehyde in reaction 4 is sterically hindered and will provide information about the ability of these catalysts to tolerate bulky groups near the reactive functionality. Catalysts 1–3 all catalyze the reduction of 4-cyanobenzaldehyde poorly (presumably due to coordination of the nitrile to the iron), so reaction 5 will examine how well the catalysts tolerate Lewis basic functional groups. Reaction 6 gives information about how active the catalysts are at reducing imines to amines.
An automated GC is allowing these reactions to be analyzed efficiently, and while we are early in the process, trends are beginning to appear. Speaking generally, catalysts with more bulky groups in the 2 and 5 positions of the cyclopentadienone (10, 11, and 12) produce products in higher yields than those bearing smaller groups. We anticipate gaining important insights into catalyst activity through this initial study.
At this point my ACS PRF award has directly impacted the research of three undergraduate students, all who have contributed to the research mentioned above. One of those students is currently in a chemistry Ph.D. program, one is employed as a chemist, and one is planning on attending a chemistry Ph.D. program. This project started as a small independent project in an inorganic chemistry course, and the funding by the ACS PRF has allowed us to explore the chemistry in much more detail. In conjunction with funding from my department, it has allowed me to continue having an active research program heavily involving undergraduate students.
Copyright © 2014 American Chemical Society