58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND251785-ND2: Elucidation of Reactions Mediated by Sulfidic Carbonate and Clay Depositions: The Search for New Organic Reactions Mediated by Natural Materials
Salvatore D. Lepore, PhD, Florida Atlantic University
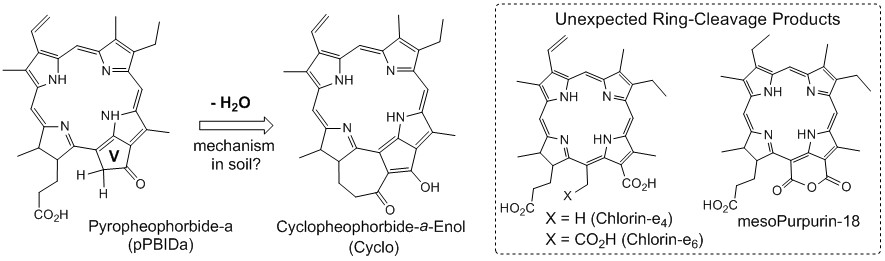
Introduction. This first phase of the project aimed to discover new reactions mediated by natural and abundant materials such as depositions of sulfidic carbonates and clays. These materials have been thought to chemically transform precursors into petroporphyrins which are sometimes used to "fingerprint" petroleum. The second phase will attempt to apply the discovered reactions to small organic compounds for potential applications in synthetic organic chemistry. Our early efforts attempted to reproduce in vitro the conversion of a series of porphyrin starting materials into their putative products. Specifically, we have spent this past year attempting to duplicate a reaction found in nature, namely the cyclization of pyropheophorbide-a free acid to give cyclopheophorbide-a-enol (Cyclo) (Figure 1). This reaction is known to occur in the sulfidic carbonate marls of Florida Bay (Louda et al., 2000).
Figure 1. Search for a sulfide-mediated mechanism to give Cyclo led to unexpected oxidative ring opening products.
Results. We have examined approximately 150 combinations and permutations at room (22-240C) or elevated (32-340C) temperatures for periods of 1-3 weeks using the following constituents singly or in various combinations as slurries in argon flushed sterile seawater: calcium carbonate, magnesium carbonate, raw and 1-20 micron ground natural aragonite, calcium bentonite, sodium bentonite, silica sand, humic acid sodium salt, calcium bentonite, sodium hydrogen sulfide (NaHS), ammonium sulfide (NH4S) and pyropheophorbide-a in both the free acid and methyl ester forms. All tests were performed in the dark. It is important to utilize ‘sterile' seawater as marine microbes have been shown to influence the degradation chlorophyll and its derivatives (Szymczak-Żyła et al., 2010). Thus far, the target compound, cyclopheophorbide-a has not been isolated though its immediate isomerization products, the epimeric chlorophyllones-a have been indicated in trace amounts.
Recent Breakthrough. In the course of our search for a sulfide-mediated mechanism leading to Cyclo, we have discovered an unexpected oxidative ring opening reaction (C-C bond breakage). Specifically, the exocyclic ring (ring V) of pheophorbide-a gave chlorin products (e4 and e6) and mesopurpurin-18 (Figure 1). These products were tentatively identified based on HPLC retention times and UV/Vis comparisons to known pigments. The yields for these initial results involving oxidative ring opening reactions were low. Scale-up attempts will be made to improve yields and provide sufficient material for mass spectral analysis to further confirm our preliminary findings.
Notably, these oxidative ring-cleavage reactions appear to have occurred in highly anoxic conditions. The reactions were flushed with argon to remove air and performed in the presence of (NH4)2S (a reducing agent). Clearly, the naturally derived sulfidic soil used in this study possesses an oxidizing property previously unknown. In addition to ring-V cleavages and/or oxidations, we have also observed macrocycle opening to yield linear tetrapyrroles called bilins.
Ongoing and Future Studies. We are presently repeating experiments with thorough quantitation to better characterize the unusual macrocycle oxidation mechanism. Additionally, the amount of argon-flushed sterile seawater in several of the to-be repeated is being increased to make more of a solution rather than a slurry, thus allowing better mixing to occur. We will also be performing several of the to-be repeated and/or new combinations with a wider range of pigment-to-reagent(s) ratios. To date, all of the pigment analyses performed thus far have relied upon HPLC-PDA methodologies which have been vigorously quality controlled versus known pigments. To this we will be adding LC-MS analyses for detailing some of the more speculative results and tentative identifications.
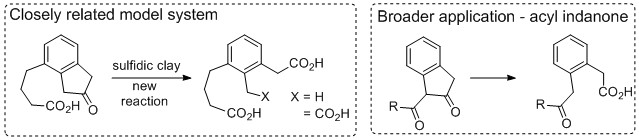
As described in our original proposal, attempts will be made to apply new reactions discovered using sulfidic clays to organic small molecules. Specifically, we will employ this new oxidative ring cleavage reaction on two small molecules systems that have similar electronic and steric properties to pyropheophorbide-a (Figure 2) Depending on the outcome, other small molecule substrates will be designed for application in this reaction to establish its generality and utility.
Figure 2. Initial application of new oxidative ring-opening reaction to organic small molecules.
References
Louda, J. W., Loitz, J. W., Rudnick, D. T. and Baker, E. W. (2000) Early diagenetic alteration of chlorophyll-a and bacteriochlorophyll-a in a contemporaneous marl ecosystem. Org. Geochem. 31 (12): 1561 – 1580.
Szymczak-Żyła, M., Louda, J. W. and Kowalewska, G. (2008) Influence of microorganisms on chlorophyll-a degradation in the marine environment. Limnol. Oceanogr. 58: 851-862.
Copyright © 2014 American Chemical Society