58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND552305-ND5: Visualization of Hydrocarbon-Surfactant Interactions at the Molecular Level
Jayne C. Garno, Louisiana State University
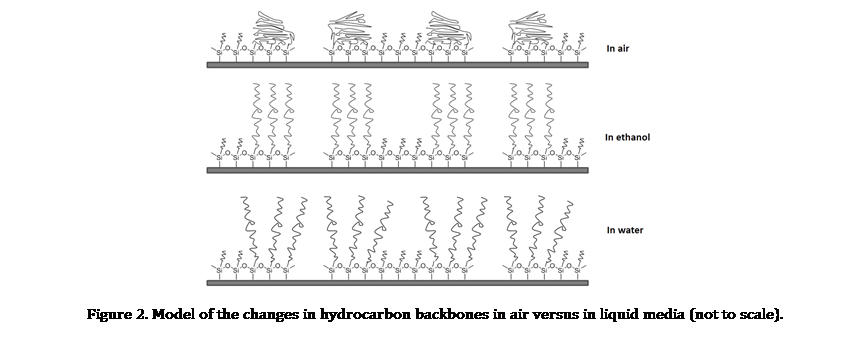
Development of Surface Test Platforms. We are working to develop protocols with scanning probe microscopy that enable visualization of hydrocarbon-surfactant interactions. Our first year has focused on developing surface test platforms which present an upright configuration of hydrocarbon chains to enable 3D visualization of molecule-surfactant interactions. Nanofabrication using particle lithography and vapor deposition of organosilanes was used to reproducibly manufacture model test platforms of hydrocarbons with well-defined composition, orientation and dimensions. When hydrocarbon nanostructures are immersed in liquid media, the dimensions and surface properties of the interface are known to change, and atomic force microscopy (AFM) experiments enable us to visualize these changes at the nanoscale. We are developing experiments to study the influence of pH, solvents, and surfactants for producing surface changes with model surfaces of designed test structures of hydrocarbon chains.
Particle lithography with organosilanes provides capabilities for surface patterning to generate ultra-small test structures. To prepare ring patterns of organosilanes shown in Figure 1, close-packed arrays of silica or latex mesospheres were used as surface masks. Vapor-phase silanes attached to the surface where water residues are present to produce periodic surface structures. When the mesosphere masks were removed, ring patterns of organosilanes with upright hydrocarbons are revealed. The patterned silane SAMs will provide ideal test
| Figure 1. Test structures of octadecyltrichlorosiloxane ring patterns imaged with contact-mode AFM in different ambient environments.
|
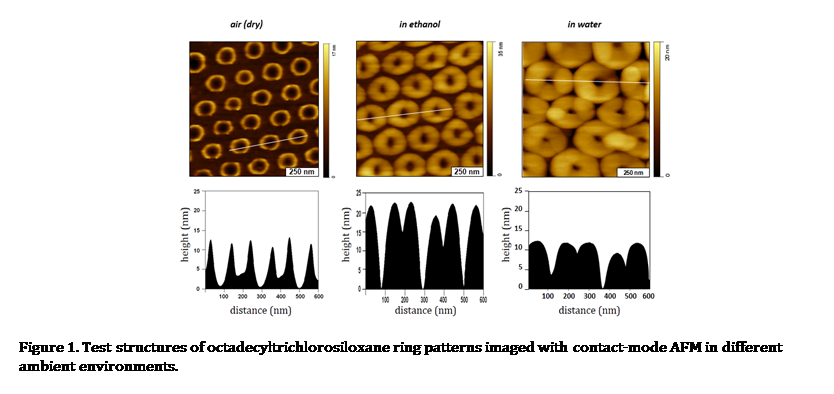 structures for in situ studies of hydrocarbon-surfactant interactions, in which
the density of surface patterns is tuned by selecting different size latex
masks.
structures for in situ studies of hydrocarbon-surfactant interactions, in which
the density of surface patterns is tuned by selecting different size latex
masks.
Educational Impact - Mentoring and Career Development.
Thus far, five female graduate students (including an underrepresented minority
student) have received graduate fellowships from this PRF New Directions award.
One of the students will complete her PhD defense for December 2013 graduation,
one student graduated in August of 2013, and the other two senior students will
graduate in 2014. Funds from this doctoral new investigator grant have been
quite valuable for advancing the PI's research program at LSU, and for
providing premiere opportunities for training graduate and undergraduate students
who are learning scanning probe techniques and learning to design experiments
to investigate the chemistry of surfaces. The funds appropriated for travel
were used to sponsor student poster presentations at nearby regional and
national conferences of the American Chemical Society. Currently, the PI's
group includes six undergraduate researchers who contribute 3-6 hours each week
to obtain research credit. Five of the undergraduates are from underrepresented
groups, and the sixth is a Caucasian female. Fellowship support for graduate
students from the ACS PRF New Directions program has bolstered the capacity of
my laboratory for sponsoring undergraduate chemistry majors for research
opportunities, which is critical to retention of students for the future
pipeline of career chemists.
Copyright © 2014 American Chemical Society











