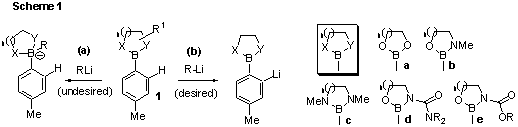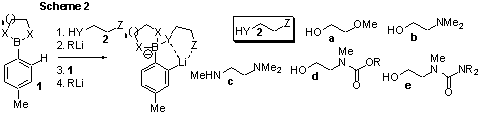58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR152231-UR1: Boron-Based Directing Groups for Directed Lithiation Reactions
J. Adam McCubbin, PhD, University of Winnipeg
A third area of
research has developed out of these projects, based on observations of
undesired product formation:
(3)
Transition metal-free Csp2-Csp2 coupling
via oxidation of aryl-vinyl boronates.
In this stage of
project (1), various derivatives (1) of 4-methylphenyl boronic acid were
prepared in order to test conditions for DoM for simple aromatic
compounds (e.g. 1a-e, Scheme 1). In addition to these, the free
boronic acid and standard derivatives (e.g. ArBF3K) were subjected
to various lithiation conditions, followed by quench with a simple electrophile
(MeI). In most cases, use of n-BuLi for lithiation at 0oC or
above resulted in decomposition products for all derivatives, which suggests
undesired nucleophilic addition to boron (via pathway (a)). Use of n-BuLi
or t-BuLi at –78oC, or lithium amides (e.g. LDA, LiTMP) at 0oC
generally resulted in no reaction (starting material recovered). Choice of
solvent (e.g. THF, heptane) had little effect on these results. We are
currently exploring the use of tri-coordinate (e.g. diethanolamine) and di-coordinate
derivatives bearing acidic hydrogens (e.g. aminoethanol) with the anticipation
that tetravalent boron may be less susceptible to nucleophilic attack, thus
favouring pathway (b).
Initial attempts
at lithiation using in-situ generated DMGs have so far been similarly
unsuccessful. Simple bifunctional alcohols and amines (2a-c,
Scheme 2) were subjected to n-BuLi at 0oC in various ether or
hydrocarbon solvents, followed by addition of a cyclic pinacol ester. At either
0oC or –78oC, further alkyllithium was added and the
solution allowed to react for one or two hours, at the same temperature or with
warming, followed by the addition of MeI. Under none of the various conditions
attempted was any of the desired product was observed, which suggests that
neither alkyllithium addition to boron nor ortho lithiation occurred. We
have prepared several more complex derivatives of the type 2d and e
for testing in this reaction, with the anticipation that the presence of amide or
carbamate groups will prove more effective at directing lithiation, leading to
successful reaction. Boronic acid derivatives other than pinacol esters have
also been prepared for testing in this reaction.
Given our lack
of success so far in developing an effective boron-based DMG, and the desire to
provide undergraduate students with the opportunity to generate some positive
results, we turned our attention to a reaction whose discovery was inspired by
some of our initial results in these studies. Based on our observations related
to alkyllithium addition to boron, we reasoned that vinylboronates (generated
by addition of a vinyl metal species to arylboronic acid pinacol esters) might
rearrange via 1,2 migration of the aryl group with the addition of an
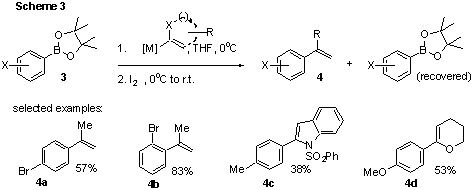
appropriate electrophile. Indeed, with the use of isopropenyl magnesium bromide
and after careful optimization of the other reaction conditions (Scheme 3), we
observed coupled products 4 in moderate yields along with unreacted
staring materials. The initial scope of the reaction was explored with a
variety of simple benzene derivatives (15 examples, e.g. 4a and b),
for which a pronounced steric effect was observed. In general, substrates with
large substituents ortho to boron afforded higher yields of 4
(with less recovered starting materials) than those with no ortho substituents.
It is also possible to subject 3 to diverse lithiated heterocycles,
followed by I2 to afford similarly coupled products (6 examples,
e.g. 4c and d). Yields observed in the heterocyclic series are so
far moderate at best, but the steric effect observed for simple benzene
derivatives has suggested a promising possibility for further yield
optimization. We anticipate that modification of the protecting group on boron
in 3 to something more sterically hindered may favour product formation
over starting material recovered.
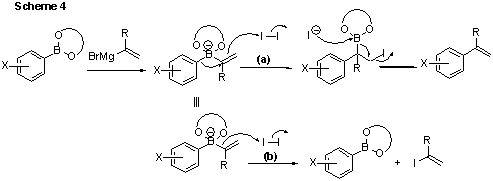
We propose two
competing mechanisms to account for the formation of the desired products and
the recovered starting material (Scheme 4). Addition of the vinyl metal species
to the pinacol ester affords a vinyl boronate, which when subjected to the
electrophile, either attacks (a) via the pi bond of the vinyl group or (b) via
the vinyl-boron sigma bond. Competition between these modes of nucleophilic
attack depends on the relative steric accessibility of each bond. Pathway (a)
leads initially to a migration product that undergoes elimination to the
coupled product
Further
investigation for the development of an effective boron-based DMG remains our
primary focus, although work in this third promising area continues.
Copyright © 2014 American Chemical Society