58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR1052216-UR10: X-ray and Neutron Scattering Studies of Proton Conduction Pathways and Dynamics in Doped Pyrophosphates
Cristian E. Botez, University of Texas (El Paso)
During this initial period we have carried out experiments related to our first objective: to accurately determine the crystal structures and their temperature evolution for the Sn1-xInxP2O7 series, identify subtle doping-induced structural features such as lattice parameter variations, tetrahedral distortions, bond-length variations, etc., and correlate these to the enhancement of the proton conductivity observed in Sn0.9In0.1P2O7. To this end, we have successfully synthesized samples with different doping levels x, from 0 to 0.18, and completed both our in-house and synchrotron x-ray temperature-resolved diffraction data collection and analysis. The synchrotron work, carried out on the X16C beamline at the NSLS, followed a successful user access proposal and included on site access and actual beamline operation by UTEP undergraduate students.
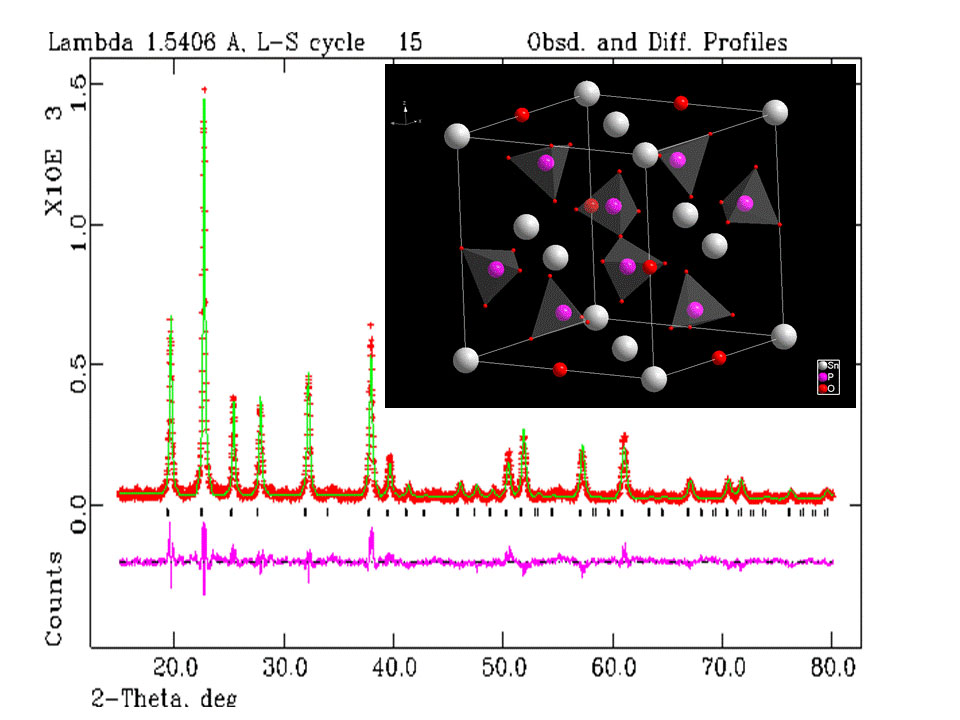
Our main findings are the following. First, we found that the sample synthesis of the Sn1-xInxP2O7 series yielded pure samples for all values of x smaller or equal to 0.12, a behavior that can be ascribed to the solubility limit of the In ions. Yet, even for x=0.14 and 0.18 we managed to identify the impurities in these highly-doped phosphates and carry out two-phase refinements of enough quality to determine the temperature dependence of their structural details. We determined the crystal structures of all samples with sub-Angstrom resolution (from Rietveld refinements as the one shown in Figure 1) and noticed that neither temperature nor doping leads to polymorphic phase transitions in any of the compounds investigated so far (i.e. the P a -3 cubic symmetry and the salient structural features are the same at all temperatures and for all doping levels).
Figure 1
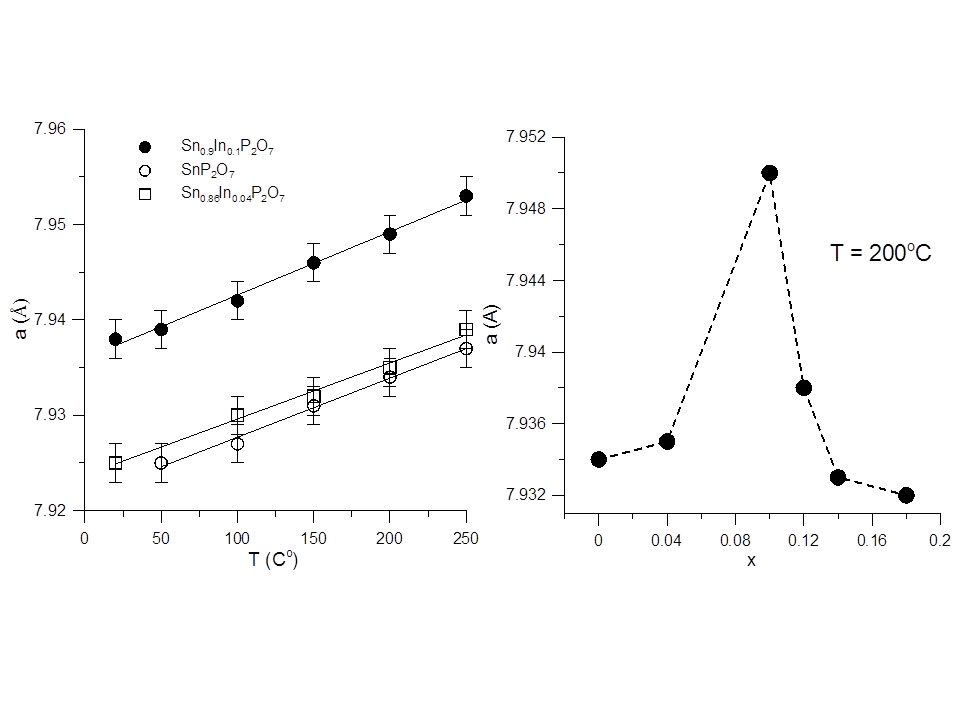
Our most important result so far is presented in Figure 2. The left panel shows the temperature dependence of the lattice parameter for samples of three different doping values obtained from refinements against temperature-resolved synchrotron x-ray data. While all the “a vs. T” dependencies are expectedly linear, the lattice parameter values for x=0.1 are at all temperatures greater than their x=0 and x=0.04 counterparts. We carried out similar measurements and analyses for x=0.12, 0.14, and 0.18, and we observed that lower values of the lattice parameter reenter upon further doping (right panel). This is a remarkable behavior and represents the first atomic-level hint of what is “special” about the x=0.1 (Sn0.9In0.1P2O7) compound, which is known to exhibit a proton conductivity at 200oC that is four times greater than any of its Sn1-xInxP2O7 series counterparts.
Figure 2
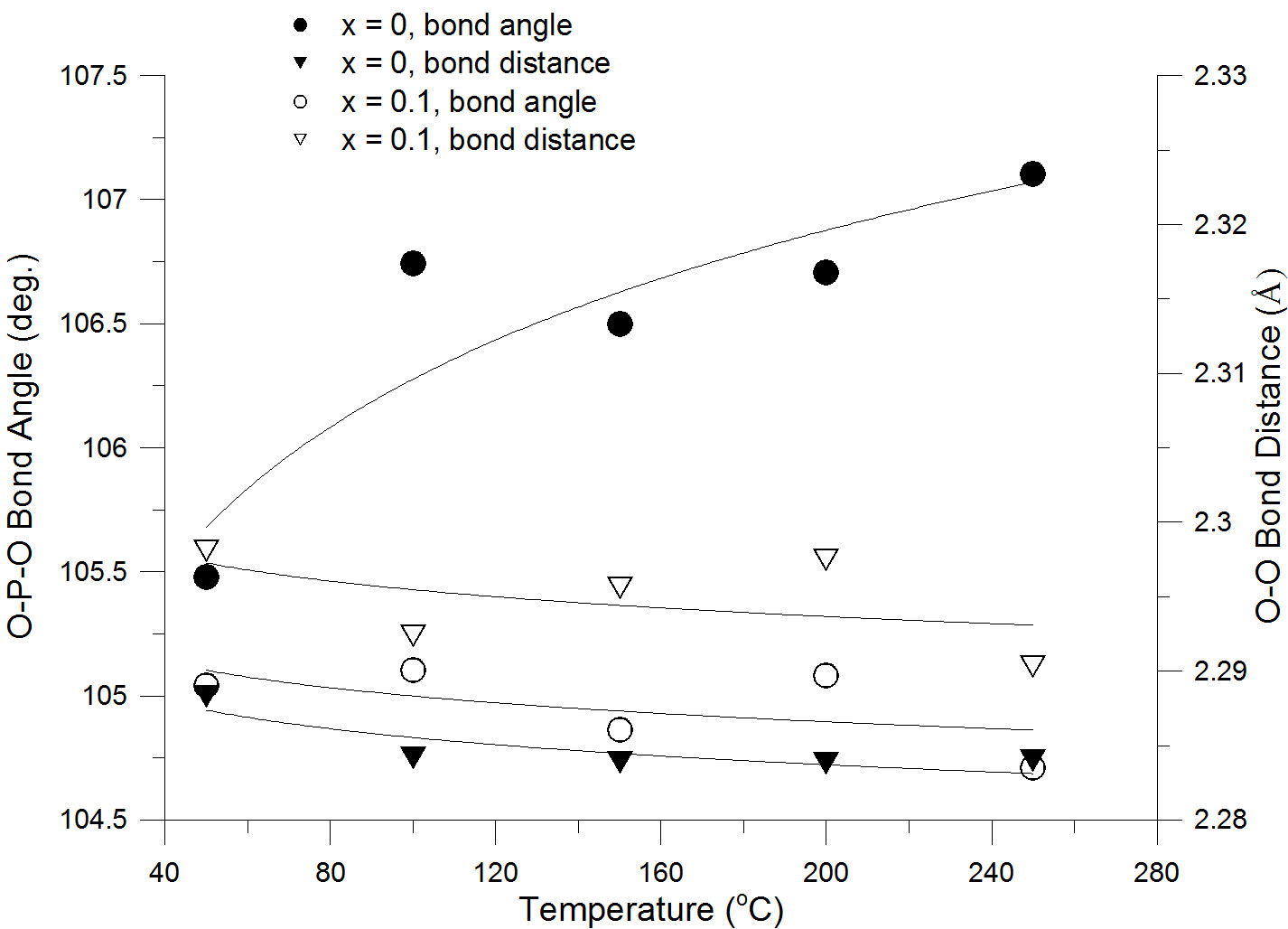
We also analyzed the temperature (T) and doping level (x) behavior of other structural features of the investigated samples. For example, Figure 3 shows the temperature dependence of selected bond angles and bond distances measured within the 40oC – 260oC interval for the Sn0.9In0.1P2O7 and SnP2O7 samples. While the bond angle for the undoped sample exhibits a slightly different temperature behavior than the one corresponding to the x=0.1 compound, the bond distance dependencies nearly overlap. It appears therefore that tetrahedral distortions are not likely to be major contributors to the proton conductivity enhancement in the x=0.1 member of the Sn1-xInxP2O7 series.
Figure 3
In addition to allowing us to carry out fundamental research, this grant has already had a very strong positive impact on the PI’s program and career development, as well as on broadening the experiences of our undergraduate students. Indeed, the grant enhanced our ability to prepare and carry out unique experiments at national facilities, offering outstanding opportunities both for the PI and for the students involved. For example, based on the research supported by this ACS-PRF grant, the PI’s newest graduate student received a scholarship from CONACyT (Mexico) to work on the project. Other undergraduate and graduate students joined the project supported by sources other than ACS PRF (e.g. by NIH’s Minority Access to Research Careers Program). In addition, the results obtained with the support of this grant will allow the PI to apply in the 2013-2014 academic year for new research funding from the Department of Energy’s Office of Basic Science. Clearly, all these aspects represent a major positive influence on the PI’s career as a researcher, mentor and instructor.
At the same time, the undergraduate students who participate in the project are receiving major benefits. Six undergraduate students actively participated in the experimental work carried out on the project with already notable results including a summer internship at Sandia National Labs to work collaboratively with University of New Mexico researchers on the sample synthesis, travel to the NSLS, training in beamline operation and temperature-resolved synchrotron x-ray diffraction experiments. In addition, our initial results obtained under this sponsorship were selected by the College of Science to be presented by one of our undergraduate students to the Advisory Board during the October 2013 meeting. Two journal articles, one in Materials Characterization and one in Materials Chemistry and Physics that acknowledge this and previous ACS-PRF support have been accepted for publication this year. Both include undergraduate students as co-authors.
Copyright © 2014 American Chemical Society