58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR452099-UR4: Mechanisms and Dynamics of Carbene Additions to Anti-Bredt Olefins
Dina C. Merrer, PhD, Barnard College
Scheme 1
Scheme 2
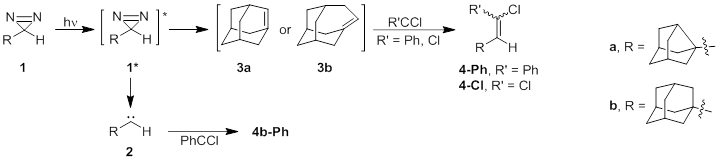
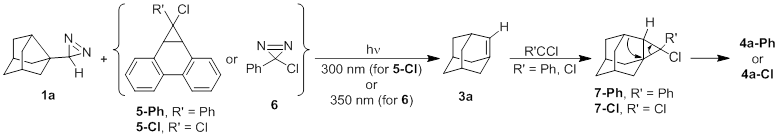
The formation
of 4a surprised us. We propose its origin via the mechanism shown in
Scheme 2. To investigate this path, we monitored the photolysis of 1a +
6 via 1H NMR spectroscopy by following the disappearance of
the diazirine proton of 1a and the appearance of the vinyl protons of E-
and Z-4a-Ph. Given the Platz group's report of a microsecond
lifetime of 3a at room temperature,3
we did not expect to see 3a by NMR, but we did investigate the
possibility of the formation (and subsequent disappearance) of adduct 7-Ph.
Careful exploration of the far upfield range (d -0.1 to +0.5 ppm) showed no appearance of the
cyclopropyl proton of 7-Ph.
(2) Martella, D. J.; Jones, M., Jr.; Schleyer, P. v. R.; Maier, W. F. J. Am.
Chem. Soc. 1979, 101, 7634-7637.
(3) Tae, E. L.; Ventre, C.; Zhu, Z.; Likhotvorik, I.; Ford, F.; Tippmann, E.;
Platz, M. S. J. Phys. Chem. A 2001, 105, 10146-10154.
(4) Tae, E. L.; Zhu, Z.; Platz, M. S. J. Phys. Chem. A 2001, 105,
3803-3807.
(5) Likhotvorik, I. R.; Tae, E. L.; Ventre, C.; Platz, M. S. Tetrahedron
Lett. 2000, 41, 795-796.
(6) Graham, W. H. J. Am. Chem. Soc. 1965, 87, 4396.
(7) Chateauneuf, J. E.; Johnson, R. P.; Kirchhoff, M. M. J. Am. Chem. Soc.
1990, 112, 3217-3218.
(8) Joshi, G. C.; N., S.; Pande, L. M. Synthesis 1972, 317.
(9) Robert, M.; Likhotvorik, I.; Platz, M. S.; Abbot, S. C.; Johnson, R. P. J.
Phys. Chem. A 1998, 102, 1507-1513.
(10) Jackson, J. E.; Platz, M. S. In Advances in Carbene Chemistry;
Brinker, U. H., Ed.; JAI Press: Stamford, CT, 1994; Vol. 1, pp 89-160.
(11) Becker, K. B. Helv. Chim. Acta 1977, 60,
81-93.
Copyright © 2014 American Chemical Society











