58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR451031-UR4: The Kinetics of Aggregating Dyes
Peter J. Collings, PhD, Swarthmore College
The Second Step of Assembly in IR-806: Equilibrium Studies
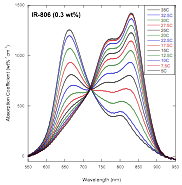
The finding of a threshold for the higher concentration process of assembly in IR-806[1] prompted us to study this more closely by conducting careful equilibrium experiments. But such experiments are problematic for the following reason. The absorption of IR-806 is quite high, meaning that absorption experiments that can follow the complete absorption spectrum must be conducted in very thin samples (25 – 75 µm). Filling and sealing these samples cannot be done in a way that guarantees that the concentration is known precisely. For this reason, the scatter in the data as the concentration is varied is considerable. However, this grant allowed us to purchase a temperature controller for the uv-vis spectrophotometer, so we decided to study the process by varying the temperature of a single concentration sample. Such a technique has been reported in the literature for a number of systems, with Ref. [2] being an example. The results were nothing less than startling, in that a near perfect isosbestic point emerged (see the figure below), providing convincing evidence that only two absorbing species are involved in this assembly process.
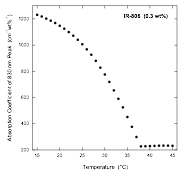
Furthermore, when the absorption due to the larger assembly is plotted versus temperature, it is clear that this process is cooperative with a specific temperature above which no assembly takes place (see figure below).
More detailed analysis of these data, with comparisons to theoretical models for assembly, is ongoing and should be completed within a couple of months. Our expectation is that we will be able to determine parameters for the assembly process that have not been measured before, such as the enthalpy change, entropy change, and degree of cooperativity.
The Second Step of Assembly in IR-806: Kinetic Studies
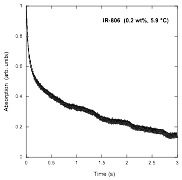
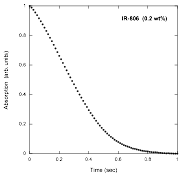
In reviewing the kinetics results reported in our annual report a year ago, it became clear that such experiments in chromonic liquid crystal systems present a challenge. Since the assembly process strongly depends on both concentration and temperature, performing experiments by varying the concentration at a fixed temperature or by varying the temperature at a fixed concentration does not achieve the goal of starting each kinetics experiment with the system in the same state of assembly. So we used the equilibrium experiments described above to find points in the concentration – temperature plane where the system is at the same point in the assembly process. The slope of these lines, 43 °C/wt%, roughly parallels the liquid – liquid crystal transition line, but more important, it allows us to conduct kinetics experiments at different temperatures starting from the same assembly state. The results of a typical kinetics experiment in which disassembly is promoted by rapid dilution are shown in the following figure.
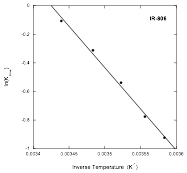
Notice that there are fast and slow processes. This agrees well with a nucleation and growth model, in which the fast process is the breakup of large assemblies into small ones close to the nucleus size, followed by the slow process in which the nuclei disassemble. Since the experiments were done at different temperatures, the rate constant for the more simple step, the break up of the nuclei, can be analyzed through the use of the Arrhenius plot shown below, where the result is that the process is governed by an activation energy of (48 ± 2) kJ/mol.
Analysis of the faster process, the breakup of the large assemblies, is more complicated and we are working on that by direct comparison to theory.
We have continued to investigate the kinetics of assembly, using our knowledge of the assembly state throughout the temperature – concentration plane so we begin each experiment with the system in the same state of assembly. A typical result is shown below, and we are finding that the analysis is a bit complicated.
Overall, the kinetic profiles are well described by a nucleation and growth model, and preliminary analysis is revealing that the rate-limiting step for assembly is the fragmentation of larger assemblies that have already formed rather than the formation of nuclei. This analysis is ongoing and will probably take many months to complete.
The Assembly Processes in Pinacyanol Acetate
We have continued to investigate the assembly behavior in the pinacyanol acetate system[3] for comparison with what we are discovering about the IR-806 system. As reported last year, the behavior is in fact quite different. If there are two steps in the assembly process, they occur simultaneously and neither process is governed by a threshold. We repeated the kinetics experiments on pinacyanol acetate using the faster instrument at Fox Chase Cancer Center, again finding that the process is too fast (over in less than 1 ms). This is what we find for chromonic liquid crystal systems formed by simple stacks of molecules, and for the first step in the assembly process of IR-806. So whatever happens in pinacyanol acetate, it does not involve a nucleation and grown mechanism to form the assemblies that spontaneously order orientationally in the liquid crystal phase. Our plan is to conduct equilibrium studies of the assembly process in pinacyanol acetate similar to the temperature studies described above for IR-806. We expect to be able to conclude whether there is cooperative behavior or not.
[1] Mills, E. A.; Regan, M. H.; Stanic, V.; Collings, P. J. J. Phys. Chem. B 2012,116,13506.
[2] Smulders, M. M. J.; Nieuwenhuizen, M. M. L.; de Greed, T. F. A.; van der Schoot, P.; Schenning, A. P. H. J.; Meijer, E. W. Chem. Eur. J. 2010, 16, 362.
[3] Rodriguez-Abreu, C.; Torres, C. A.; Tiddy, G. J. T. Langmuir 2011, 27, 3067-3073.
Copyright © 2014 American Chemical Society