58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI451008-UNI4: Specific Ion Effects on the Interfacial Properties at the Hydrophobic/Aqueous Interfaces
Yanjie Zhang, PhD, James Madison University
Specific ion effects on the physicochemical properties of aqueous processes such as polymer and protein aggregation, protein folding, and enzyme activity follow an empirical trend that is called the Hofmeister series. Typical orders for the ability of anions and cations to salt proteins out of solution are as follows:
CO32- > SO42- > S2O32- > H2PO4- > F- > Cl- > Br- > ~NO3- > I- > SCN-
NH4+ > Cs+ > Rb+ > K+ > Na+ > Li+ > Ca2+ > Mg2+
Although the Hofmeister effects are general phenomena in many different systems, the molecular level mechanisms of the Hofmeister series are not well understood. The effects of anions on uncharged systems are stronger than cations. Therefore, the studies of the effects of cations are more difficult than similar studies of anions. In our study, the effects of a series of fourteen cations on the phase behaviors of a triblock copolymer, poly-(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO) were investigated to explore the mechanisms of cation-macromolecule interactions.
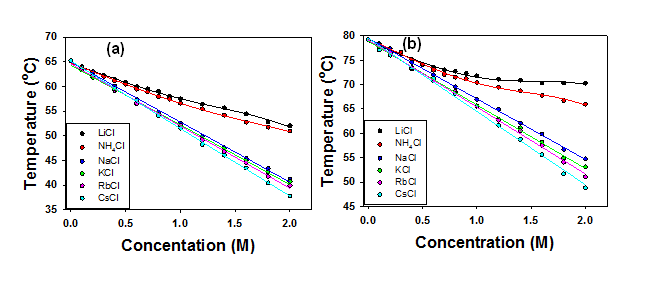
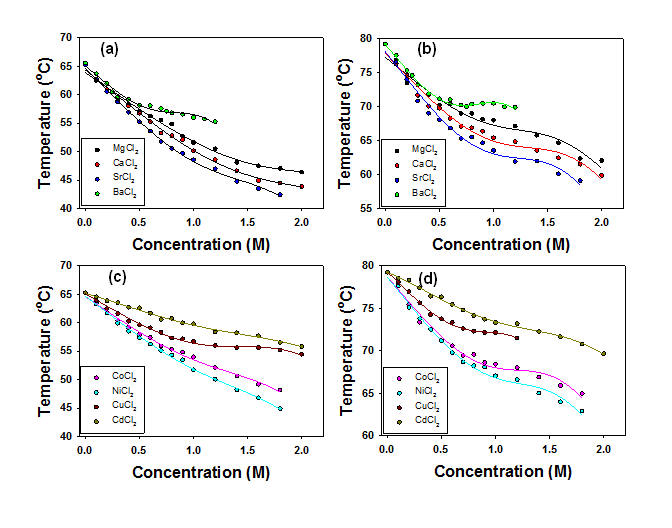
The phase transition temperatures of a triblock polymer, L44, as a function of salt concentration are shown in Figure 1 and 2. Clearly, we are not able to isolate cations from a salt solution. Therefore, the effects of salts we observed herein are joint effects of cation and the Cl- anion. The phase transition temperature of L44 decreases linearly as salt concentration increases in the presence of NaCl, KCl, RbCl, and CsCl. The plots for LiCl, NH4Cl, and all of the salts of divalent cations employed show clear curvatures. In aqueous solution, cations could interact with the polymer in three ways distinguished by how cations share water molecules with the polymer. First, cations could bind directly to the polymer via electrostatic interactions between the partial negatively charged oxygen in the polymer chains and the cations. This direct binding of cations will introduce extra charges to the hydrated polymer and the phase transition temperature increases. The cations with high charge densities such as Li+ and divalent cations have a tendency to associate with the polymer via electrostatic interactions. If the hydration of a certain cation is relatively weak, it could shed its hydration water and bind to the oxygen in the polymer chain. The binding of cations to oxygen should reach saturation at high cation concentrations. Second, cations could polarize the water molecules that are directly hydrogen-bonded to the oxygen in the polymer. When a cation in the vicinity of a water molecule that is directly hydrogen-bonded to the polymer, the cation draws electron density to the oxygen and leaves the hydrogen in the water molecule more positively charged. The cation in turn strengthens the hydrogen binding of the water molecule to the polymer's oxygen. A water molecule in the first hydration layer serves as a bridge between the polymer and the cation. This mechanism makes the dehydration from the polymer more difficult and increases the phase transition temperature. Third, the interactions between the cations and the polymer could be mediated by two water molecules bridging the cation and the oxygen in the polymer. This mechanism actually weakens the hydrogen bonding between water and the oxygen in the polymer chains. Therefore, the hydration water around the polymer is easier to remove and the phase transition temperature decreases. Two forms of polarization of hydration water around the polymer's oxygen should coexist when a cation is present in the vicinity of the polymer. The effect of water polarization by cations should be a net result of the competition and balance between these two forms of polarization. Moreover, the magnitude of these two forms of polarization of water molecules should be linearly proportional to the cation concentration in the solution. The ability of a cation to polarize water molecules should correlate to the hydration thermodynamics of the cation.
Figure 1. (a) The first and (b) the second step of the phase transition for L44 plotted vs. salt concentration for monovalent cations.
Figure 2. (a) The first and (b) the second step of the phase transition for L44 plotted vs. salt concentration for alkaline earth metals; (c) The first and (d) the second step of the phase transition for L44 plotted vs. salt concentration for transition metals.
In the discussions on cation effects, Na+ is employed as a benchmark to determine if a particular cation has salting-our or salting-in effects as compared to Na+. Our results indicated that strongly hydrated cations such as Li+ have salting-in effects on the polymer, while weakly hydrated cations have salting-out effects on the polymer. In the first step of the phase transition, almost all the divalent cations show salting-in effects except for Sr2+ and Ba2+. In the second step of the phase transition, only Mg2+ and Cd2+ exhibit salting-in effects while all other divalent cations show salting-out effects. Our studies suggest that the hydration of cations plays a key role in determining how cations interact with the polymer. Our results have been published in J. Phys. Chem. B.
Copyright © 2014 American Chemical Society