58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR351147-UR3: Control of Selectivity in Intramolecular Carbon-Hydrogen Bond Activations by Transition Metal Complexes
Shouquan Huo, Ph.D., East Carolina University
Over the last grant period, our group has been actively engaged in the proposed research, having produced many results. Specifically, the progress includes (1) investigation on the selective C-H bond activation, C-N bond cleavage, and selective acylation process in the reaction of N-isopropyl-N-phenyl-2,2'-bipyridin-6-amine with K2PtCl4, (2) control of selectivity in cycloplatination of N-methyl-N-phenyl-6-(1H-pyrazol-1-yl)pyridin-2-amine, and (3) design and synthesis of phosphorescent tetradentate bis-cyclometalated C^C*N^N-coordinated platinum complexes through forced double cycloplatination reaction.
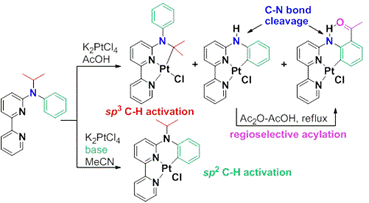
Selective C-H bond activation, C-N bond cleavage, and selective acylation process in the reaction of N-isopropyl-N-phenyl-2,2'-bipyridin-6-amine with K2PtCl4
Investigation on the reaction of N-isopropyl-N-phenyl-2,2'-bipyridin-6-amine with K2PtCl4 was a continuation of research from the previous grant period. The selective C-H bond activation of N-alkyl-N-phenyl-2,2'-bipyridin-6-amine promoted by Pt(II) was complicated by the low selectivity of sp2 C-H bond activation in acetonitrile and low yielding of sp3 C-H activation in acetic acid. The use of a base was found to effectively suppress the competing sp3 C-H bond activation in acetonitrile, improving the selectivity of sp2 C-H bond activation from 70% to 99%. In the reaction in acetic acid, the low yield was due to the competing C-N bond cleavage. The use of a base reduced the C-N bond cleavage but not completely. The reaction of N-tert-butyl-N-phenyl-2,2'-bipyridin-6-amine with K2PtCl4 in acetic acid produced the cyclometalated complex with complete C-N bond cleavage and its acylated derivative. These results indicated that the C-N bond cleavage might proceed via heterolytic C-N bond dissociation. The acylation following the C-N cleavage in the reaction in acetic acid is regioselective. Further experiments showed that the reaction of N-phenyl-2,2'-bipyridin-6-amine with K2PtCl4 in acetic acid produced the cyclometalated complex, while the reaction in a mixture of acetic anhydride and acetic acid produced the acylated cyclometalated complex. X-ray crystal structure revealed a strong intramolecular H-bonding in the acylated complexes. We are currently designing new experiments to gain an insight into the mechanism of this regioselective acylation reaction.
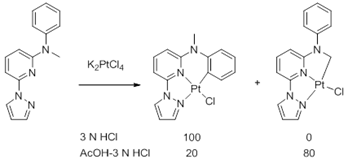
Control of selectivity in cycloplatination of N-methyl-N-phenyl-6-(1H-pyrazol-1-yl)pyridin-2-amine
As a continuous efforts to gain more understanding on the control of selectivity in the competing intramolecular sp2/sp3 C-H bond activation process, a specially designed ligand, N-methyl-N-phenyl-6-(1H-pyrazol-1-yl)pyridin-2-amine, was prepared and its reaction with K2PtCl4 was investigated. To our surprise, the reaction didn't proceed at all in acetonitrile, the conditions that favored an sp2 C-H bond activation as e previously reported in the reaction of N-methyl-N-phenyl-2,2'-bipyridin-6-amine. Furthermore, the reaction in acetic acid gave a mixture resulted from both sp2 and sp3 C-H bond activation, while the reaction of N-methyl-N-phenyl-2,2'-bipyridin-6-amine in acetic acid resulted in exclusively sp3 C-H bond activation. Further investigation showed that the exclusive sp2 C-H bond activation could be achieved when the reaction was carried out in hydrochloric acid. The selective sp3 C-H bond activation was observed when the solvent was changed to a mixture of hydrochloric acid and acetic acid, however, the selectivity was only 80%. It was confirmed that the isomerization between sp2 and sp3 C-H activation products readily proceeded in refluxing hydrochloric acid-acetic acid and reached to the equilibrium within a few hours. These results indicated the formation of the product of sp3 C-H activation was thermodynamically controlled.
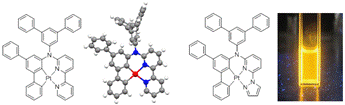
Phosphorescent tetradentate bis-cyclometalated C^C*N^N-coordinated platinum complexes
The competing C-H bond activation in the cycloplatination of tridentate CNN ligands led us to an ultimate design of the tetra dentate cyclometalating ligands to form a C^C*N^N-coordinated platinum complex through a forced consecutive double sp2 C-H bond activation. The coordination geometry of the complexes was confirmed by the X-ray crystallography. One of the complexes emitted intensely yellow phosphorescence at room temperature in deoxygenated solution.
Over the last two years, my research has been mainly supported by the PRF grant. The students' involvement has been very substantial and has proved to be beneficial to both the students and my research. Both undergraduate and graduate students have enjoyed working on the project. They are especially fascinated by the controls in the competing C-H bond activation, a fundamental thermodynamic and kinetic chemical problem that they could only learned from the chemical classes and textbooks previously. Three undergraduate students and one graduate received stipends and worked as full time research assistants in the past summer. Some of them are expected to present their results at either ACS meetings or the National Conferences for Undergraduate Research.
Copyright © 2014 American Chemical Society