58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI451842-DNI4: Hydrazone-Based Rotary Switches as Proton-Relay Systems
Ivan Aprahamian, Dartmouth College
Proton relay and transport play an important role in many applications related to the fields of petroleum and alternative energy, such as, electrocatalysis, hydrogen sensors, supercapacitors, polymer electrolyte membrane (PEMs) fuel cells, and so on. With this proposal we aimed at gaining a fundamental understanding of how to control and exploit the vehicle-type proton transfer in imidazole-containing hydrazone-based rotary switches. The end goal of this research program is the development of highly conductive PEMs that operate at high temperatures and under anhydrous conditions making them viable and cheaper alternatives to currently used PEMs that are based on perfluorinated ionomers.
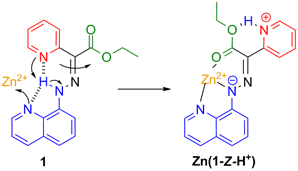
Scheme 1. Zinc(II)-initiated coordination-coupled deprotonation and switching of 1.
We have previously shown that the molecular switch, 1, can act as a tridentate ligand. The coordination of Zn2+ with 1 results in i) deprotonation of the hydrazone NH proton, ii) protonation of the pyridyl ring, and iii) subsequent E/Z isomerization of the switch (Scheme 1; Zn(1-Z-H+)). This novel switching mechanism, which relies on coordination-coupled deprotonation (CCD) is reminiscent of the binding of transition metals with protein residues, which often leads to proton displacement and subsequent relay. Significantly, similar to the biological processes the coordination with Zn2+ with 1 drastically changes the pKa of the system. Here we elaborate how the ACS PRF funding enabled us to take advantage of the CCD process and associated pKa changes in controlling the switching of hydrazone-based systems through proton relay.
Results and Discussion
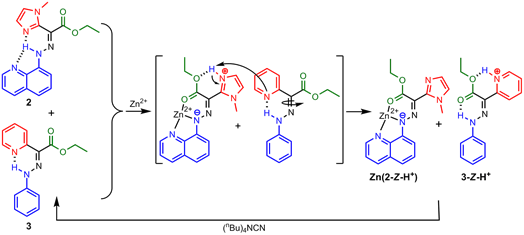
Scheme 2. The switching cascade of 2 and 3 through CCD initiated proton relays.
We have made strides in taking advantage of the CCD process to make a hydrazone system that once switched acts as the power source for another molecular switch (Scheme 2). In order to achieve this feat we replaced the pyridyl ring in the hydrazone switch with a methyl imidazolyl one (2), which is known to be involved in CCD initiated proton relays in biological systems. We then showed that once the imidazolium ring is formed by the zinc(II) initiated CCD process (Zn(2-Z-H+)), it can itself act as a proton source that can activate a different non-coordinating pyridine-containing hydrazone switch (3) through proton relay. The crucial step in the switching process is the lowering of the pKa of the imidazolyl ring in 2 upon binding with zinc(II), which results from the electrostatic repulsion between the metal cation and the imidazolium ring. This pKa modulation explains how imidazole, which has a pKa of 7 can protonate pyridine, which has a pKa of 5. However, this still does not explain why proton relay does not happen in the original system, Zn(1-Z-H+).
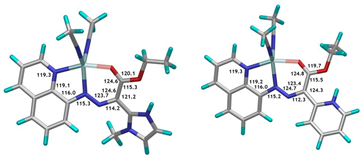
Figure 1. DFT (B3LYP/LACV3P**++) calculated structures of Zn(2-Z-H+) (right) and Zn(1-Z-H+) (left).
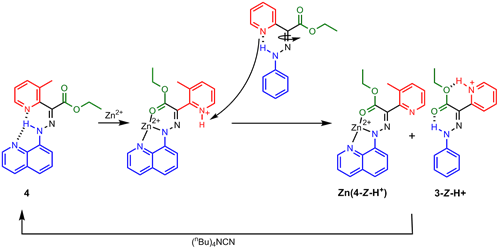
In order to understand why this is so we conducted DFT calculations and obtained the optimized geometries of Zn(1-Z-H+) and Zn(2-Z-H+) (Figure 1). As it turns out, the pyridinium proton is involved in a very strong intramolecular H-bond as a result of the planarity of the system, which precludes it from being involved in proton relay. On the other hand, the methyl group on the imidazolium ring prevents it from adapting a co-planar conformation; hence, its proton is very weakly H-bonded, making it available for proton relay! In order to test this hypothesis we synthesized a methyl-pyridyl containing switch (4) and showed that this system canperform CCD initiated proton relay (Scheme 3). This result shows that both electrostatic repulsion and conformational freedom are required for the proton relay to take effect.
Scheme 3.The switching cascade using the methyl-pyridyl containing switch 4.
This multistep switching cascade is an early example of a dynamically switched compound acting as the input to another. This is somewhat reminiscent of the biological molecular motor ATP synthase, which through its mechanical operation produces the fuel to power other biological machines. This is a first step towards the ultimate goal of this project; using the imidazole-containing hydrazone switches in vehicle-type proton transfers.
Scheme 4. The locking of 5 through a H-bond.
Recently we started studying the coordination of the imidazole containing switch (5) with zinc(II), which also leads to CCD; however, the coordination does not lead to switching (Scheme 4)! We rationalized this result with the existence of a very strong H-bond between the imidazole N-H proton and the ester group in the rotor part of the switch. This H-bond “locks” the imidazole ring in place and prevents it from rotating upon coordination. We have tried to use various H-bond acceptors (e.g., F‒, CN‒, urea, etc.) as additional inputs to “unlock” the H-bond; however, our attempts were not successful. We are now looking into using the proton that is being released into the environment through CCD, in different catalytic cycles so they can amplify the signal obtained upon zinc(II) coordination.
Outlook
Our goal for the upcoming year is to incorporate the hydrazone switches into polymers in order to assess their potency in relaying protons in bulk materials.
Copyright © 2014 American Chemical Society