58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND752339-ND7: Mechanical Control of Molecular Encapsulation
Byron W. Purse, PhD, San Diego State University
The goal of this project is to test our hypothesis that polymer-appended molecular capsules will release and take up small molecules in response to mechanical forces, providing a new and orthogonal control for molecular encapsulation with prospective applications in chemical compartmentalization and materials science. A prerequisite for this supramolecular mechanochemistry is that the employed molecular capsules have unusually high kinetic stability; guest exchange must be induced mechanically from capsules filled with small molecules that are different from those preferred at equilibrium. Success also requires synthetic methods to prepare homogeneous batches of polymer-appended capsules with low polydispersity. Our research plan is to develop syntheses for polymer-appended capsules of sufficient kinetic stability, methods to load them with guest molecules in a kinetically trapped state, purify them, and then use ultrasonication to correlate the forces required for mechanically induced guest release to chemical structure.
The first year of funding has been used to provide materials and supplies for the project, plus support for two Ph.D. students, Jennifer Chapin (supported as a GRA) and Stephen Moss (supported mostly as a GTA). With two students involved, we are simultaneously pursuing the project goals using hydrogen bonded molecular capsules and capsules assembled using metal–ligand interactions. Significant goals remain for this, the second year of funding, but major milestones have been met in the synthesis of functionalized molecular capsules for polymer attachment, the development of capsule loading conditions, and the purification of the kinetically trapped encapsulation complexes without significant guest molecule release.
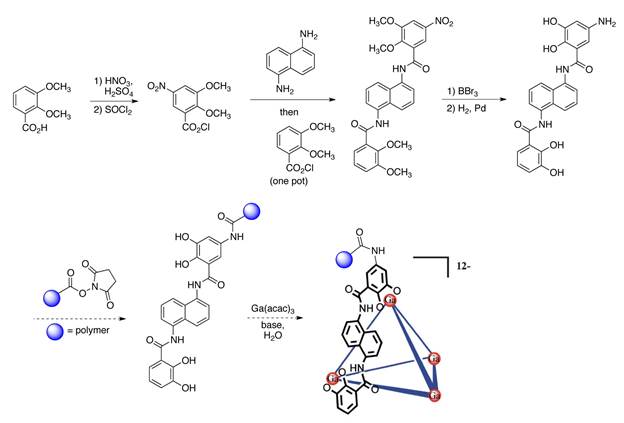
Referring first to the metal–ligand coordination capsules, we have successfully developed a synthetic route to new, monofunctionalized ligands for a M4L6 capsule that, in its unfunctionalized form, is known to have sufficient kinetic stability (Scheme 1). The amino groups of these ligands will be the reactive handles used to attach polymers. We are currently working on attaching the polymers to these ligands and will investigate the mechanochemical guest exchange from these capsules in the second year of the project.
Scheme 1
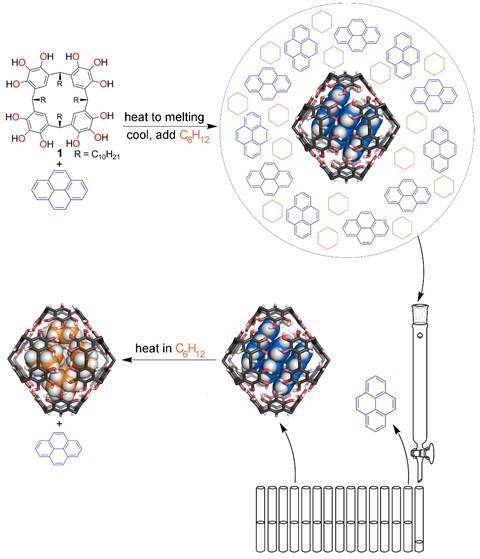
For the second approach using hydrogen bonded capsules, we have simultaneously worked on optimizing conditions for appending appropriate polymers for mechanochemistry and examined conditions suitable for purifying the capsules with kinetically trapped guest molecules and releasing those guests at a later time, on cue. Monofunctionalization of the capsules is challenging synthetically, and the amphiphilic character of the molecules makes purification difficult. We have found that synthesizing the pyrogallol[4]arene (1 in Scheme 2, made by acid-catalyzed condensation of pyrogallol with an aldehyde) with one differentiated R group using mixtures of aldehydes leads to product mixtures that are intractable to purification. Instead, we have found more encouraging results with adding a reactive handle to the end of all four R groups (the product is easier to purify) and then differentiating them by reaction with a substoichiometric amount of reagent. We are carrying this work forward for polymer attachment.
Scheme 2
In work carried out at the same time, we have found that pyrogallol[4]arene capsules can be loaded to produce kinetically trapped encapsulation complexes by mixing the pyrogallol[4]arene with pyrene, heating above the melting point of pyrene, and then allowing the mixture to cool. The loaded capsules can then be dissolved in cyclohexane, a solvent that completely displaces the pyrene as guest at equilibrium, but the activation energy needed to reach this equilibrium is not available at room temperature (DG‡ = 27 kcal/mol at 343 K). For that reason, it is possible to purify the capsules using gel permeation chromatography to separate them from any free pyrene that is present in solution (Scheme 2). At a later time of the chemist's choosing, the capsules can be induced to release their bound pyrene, either by simply heating the solution so that equilibrium (i.e. with cyclohexane bound as guest) is established or by the addition of hydrogen bonding solvents that interrupt the assembly. This result is, to the best of our knowledge, the first example of the purification of a kinetically trapped, hydrogen bonded molecular capsule of any design, followed by subsequent, induced guest release. A manuscript describing this result has been submitted for publication. This method will be useful for purifying the guest-filled, polymer-appended capsules currently under development.
In related work, we have shown that these hydrogen bonded capsules can protect alkene-containing guests from external, reactive species. Thus, an avenue is available for mechanically induced chemical reactions through interruption of compartmentalization. We are pursuing this research objective in the second year of funding.
In summary, during the first year of project funding, we have completed many key steps needed to create the polymer-appended capsules and have demonstrated the required stability and purification of the hydrogen-bonded assemblies. The funding has supported the training of two Ph.D. students, led to one submitted manuscript, and been discussed by the PI at invited lectures. The second year of this New Directions award will be used to complete synthetic work and make initial studies of the mechanochemistry, leading to publishable outcomes and the groundwork to seek larger, programmatic funding sources. As such, this grant is advancing the training of students and the career of the PI.
Copyright © 2014 American Chemical Society