58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI452125-DNI4: Watching Single Catalyst Molecules in Action
Randall H. Goldsmith, PhD, University of Wisconsin (Madison)
The goal of this proposal is to use fluorescence microscopy to make novel mechanistic measurements on individual catalyst molecules. Consequently, there are two elements to making such measurements, the building of instrumentation to perform the measurement, and the synthesis of appropriate catalyst molecules as target systems. Purification was a particularly difficult aspect of the latter category. Progress was made in both efforts.
1. Single-molecule Confocal Fluorescence Microscope
Over the past year, a home-built single-molecule confocal fluorescence microscope was constructed on a Nikon Eclipse frame. Multiple excitation colors were included for the monitoring of different fluorescently labeled functionality. A detection line was constructed to enable rapid switching between fast single-pixel detection to allow the high-detail monitoring of a single catalyst molecule, and detection on a multi-pixel EMCCD array, to allow multiple catalysts to be simultaneously monitored. Software for acquiring and processing individual photon counts was designed and implemented. A piezo-stage scanning algorithm was also implemented to allow characterization of the microscope's point spread function, a necessary diagnostic tool.
2. Synthesis of Fluorescently Labeled Catalysts
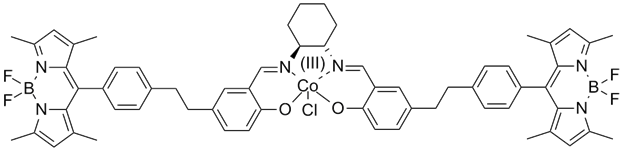
Two families of organometallic catalysts are being explored. The first is a series of cobalt salen complexes known to efficiently carry out the kinetic resolution of epoxide enantiomers (Jacobsen's Catalyst). These catalysts possess a red absorption feature that has an intensity that is tightly linked to the spin state of the cobalt, which is in turn linked to the coordination environment of the cobalt center. In a non-coordinating solvent, such as dichloromethane, the absorption feature persists, but it is absent in a coordinating solvent like tetrahydrofuran. Our goal was to attach a fluorescent molecule to the salen Co catalyst such that the fluorescence of the fluorophore would be quenched when the catalyst was not ligated by a substrate molecule, but would brightly fluoresce if it had bound a substrate epoxide. Consequently, we had hoped to monitor the coordination state of individual catalyst molecules in real time, and ultimately learn how multiple salen Co complexes interacted to catalyze the desired reaction.
Two molecules were synthesized, as shown below. The Bodipy-salen complex without the t-butyl groups was synthesized first due to its requiring of a simpler synthetic pathway. However, upon examination via absorption spectroscopy, this complex did not show the desired spectral feature, leading us to conclude that the distortion of the complex as a result of these t-butyl groups is critical to the complex's electronic structure. Second, we synthesized the complex with these groups. These complexes showed the desired feature and the expected dependence on solvent ligation strength. However, no fluorescence quenching was observed, due to the poor spectral overlap between the Bodipy fluorescence and the catalyst absorption. The synthesis of a third generation of catalyst with a Bodipy with red-shifted emission spectrum is in progress.
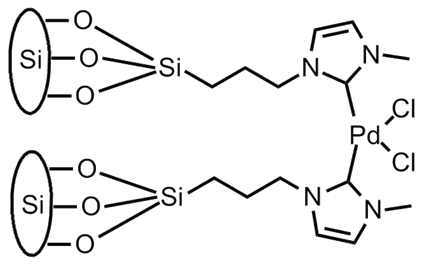
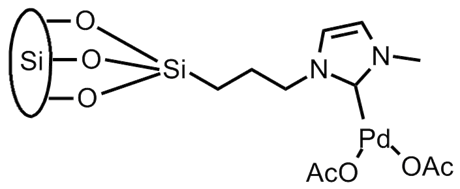
We also began the synthesis of several surface-supported palladium complexes, as shown below. We have focused on palladium coordinated via N-heterocyclic carbene (NHC) ligands as these have been shown to provide a chemically stable surface anchor and to create an extremely effective catalyst species. The synthesis of two surface-supported NHC catalysts were attempted. The bis ligated Pd species, synthesized via initial surface immobilization of the NHC salt followed by stirring with palladium acetate under mild conditions produced a surface-supported species that is catalytically competent toward the Suzuki cross-coupling reaction. In contrast, our attempts to synthesize the mono-ligated palladium species have not yet succeeded. Our use of silver oxide as a transmetallating agent has proved unsuccessful despite literature precedent and multiple attempted conditions. We continue to try new synthetic strategies.
In addition, initial examinations of “pure” solvents showed a substantial amount of fluorescent impurities, even before fluorescently labeled catalyst molecules were added. These impurities were seen even in higher spectroscopic and HPLC grades. Multiple traditional means of purification were attempted, including distillation of the solvents from a variety reagents and passage through various stationary phases. Ultimately, pure organic solvents were obtained after the construction of a “photobleaching box,” a chamber lined with multiple high-power blue LED's. Continual bleaching of solvents for multiple days after simple distillation allow us to routinely produce toluene and alcohol solvents essentially free of fluorescent impurities. Ethers and chlorinated solvents are not cleaned under these conditions, likely due to the initiation of some photochemistry that produces additional impurities.
In summary, our efforts in the past year have resulted in our building a solid foundation for our single-molecule mechanistic experiments. Instrumentation and purification issues have been largely resolved, and a family of synthesized chemical targets is nearly ready for examination. We expect next year to include observations of individual working catalyst molecules.
The progress reported was primarily accomplished by a postdoctoral researcher. This researcher arrived in my group with a primarily synthetic organic background. During his time in the group supported by a PRF DNI award, he has received valuable training in organometallic chemistry and photochemistry. This experience has made him the go-to source in the group for advice on preparing organometallic species and in experimental design for single-molecule experiments.
Copyright © 2014 American Chemical Society