58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR151819-UR1: Phosphorus-Hydrogen Activation Using Alkynylmetal Complexes: New Methodology for the Preparation of Metallopolymers
Robert A. Stockland, Bucknell University
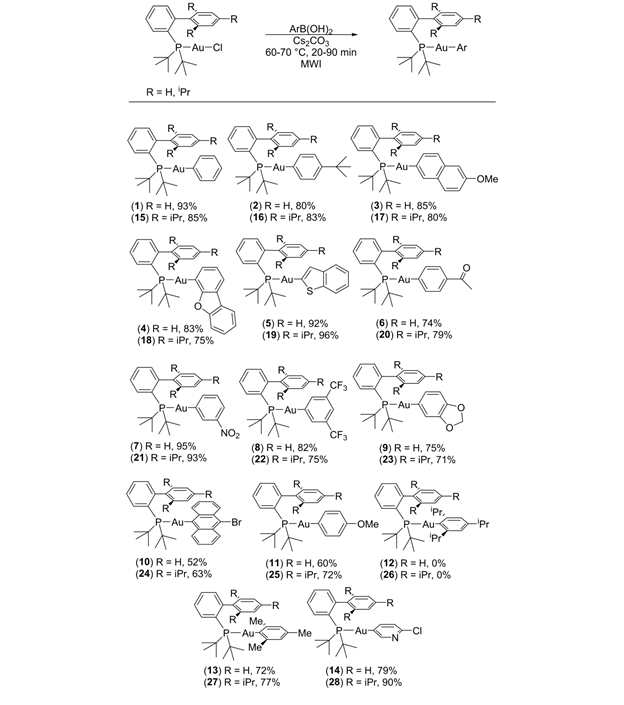
During year 1 of this project, our attention was focused on the synthesis of the organogold precursors that were needed for the preparation of the metallopolymers. In the beginning, we used alkynylgold compounds as the gold source for the new materials; however, we discovered that the aryl groups in arylgold compounds were significantly more labile and the P-H activation reactions that generated/capped the metallopolymers reached completion rapidly when using arylgold substrates. Naturally our attention shifted to the use of arylgold compounds. In contrast to the well-established synthesis of alkynylgold compounds, the preparation of arylgold species can be a bit challenging. Historically, arylgold compounds were prepared by treating chlorogold compounds with Grignard or organolithium reagents at low temperatures.1,2 This methodology does not tolerate substrates bearing electrophiles and can be operationally challenging.3 Recently, Gray has reported the use of arylboronic acids for the generation of arylgold compounds.4 The conditions required by his approach were mild, and high yields of the arylgold compounds were routinely obtained. Since these arylation reactions could take days to reach completion, we decided to try and devise a microwave assisted approach to the chemistry. After some experimentation, we developed a method for the rapid arylation of chlorogold compounds.5 Our microwave assisted approach reduced the reaction time to 20-90 minutes. This was a significant advancement for us and enabled my students to make a host of arylgold compounds (Table 1). All of the compounds were isolated by column chromatography (basic alumina) as white solids. As shown in Table 1, the chemistry tolerated a wide range of electronic and steric variation. These compounds will be used as capping agents in the metallopolymer portion of the project.
Table 1. Preparation of arylgold compounds. aReaction conditions: JohnPhosAuCl (0.19 mmol) or tBuXPhosAuCl (0.15 mmol), ArB(OH)2 (2 equiv), Cs2CO3 (2 equiv), 1.5 mL THF or iPrOH (for 10,13,24,27), 60-70 °C, 20-90 min, focused microwave reactor. bIsolated yields.
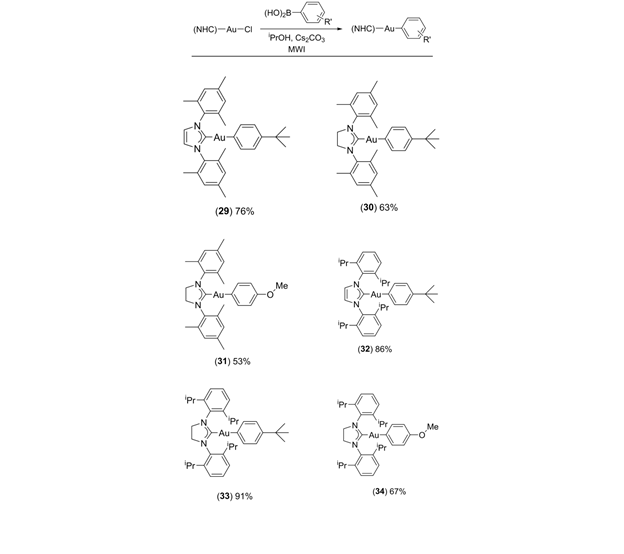
With the phosphine ligated arylgold compounds in hand, we turned our attention to N-Heterocyclic carbene derivatives. These compounds can exhibit unique reactivity in gold catalyzed reactions.6,7 To our delight, several (NHC)AuCl precursors were readily transformed into arylgold compounds using our approach (Table 2). Following isolation by column chromatography (basic alumina) the (NHC)AuAr compounds were all white solids that were quite stable to moisture and atmosphere for extended periods of time.
Table 2. Synthesis of NHC ligated arylgold compounds. aChlorogold precursor (0.32 – 0.37 mmol), 2 equiv arylboronic acid, 2 equiv Cs2CO3, 50 cC, 20 min, iPrOH (1.5 mL), microwave irradiation. b Isolated yields.
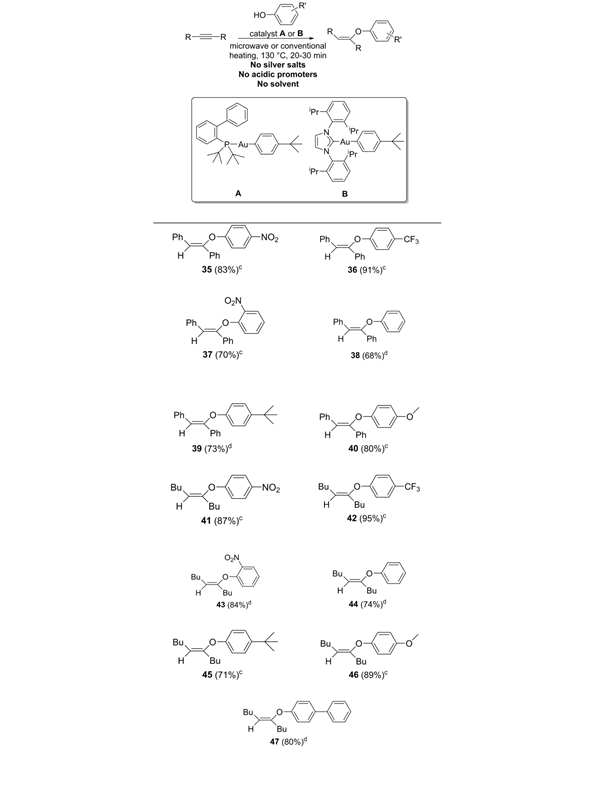
One of the reactions we proposed to investigate as part of this award was the hydrophenoxylation of internal alkynes. While the gold-catalyzed addition of primary alcohols to terminal and internal alkynes has been well established, the development of a successful catalyst for the addition of phenols has been much more challenging. Successful examples are quite rare.8,9 Since we had the arylgold compounds “in-hand” for the materials part of the project, we investigated their effectiveness to promote the addition reaction. After some exploratory experimentation, we discovered that these single-component compounds could promote the hydrophenoxylation reaction without the addition of silver salts, acidic promoters, or even solvent. Furthermore, both microwave and conventional heating successfully promoted the reaction. Given the current interest in the development of silver-free gold catalysts10-11 and sustainable organic reactions,12 this rapid hydrophenoxylation reaction should be of interest to the synthetic community.
Table 3. Hydrophenoxylation of internal alkenes. aAlkyne (0.28 mmol), phenol (0.56 mmol), 130 °C, 20 min, no solvent. bIsolated yields. cmicrowave heating with A as the catalyst (14.0 mmol, 5%). dconventional heating with B as the catalyst (14.0 mmol, 5%).
During the first phase of the project, I was fortunate to have nine very talented undergraduates working on the chemistry (two were directly funded by the PRF award). Marcia Richard, Kyle Reese, and Heather Lenker recently graduated with degrees in chemistry. Marcia is currently working for ExxonMobil in New Jersey, Kyle is attending graduate school at the University of Pittsburgh, and Heather is attending dental school at the University of Philadelphia. Erica Miller (BS Chemistry '16), Kevin Garcia (BS Chemistry '16), Rosa Ciccarelli (BS Chemistry '16), Erin Holahan (BS Cell Biology/Biochemistry '14), Victoria Resh (BS Animal Behavior '14), and Aakash Shah (BS Biology '16) all worked on the hydrophenoxylation chemistry and are current students at Bucknell. Several of these students will be traveling to New Haven to present their chemistry at the Northeastern Regional ACS meeting in October 2013.
Summary: During this initial phase of the project, we have prepared a range of arylgold compounds for use a precursors for the generation of metallopolymers. In addition to serving as end-capping reagents, several of these compounds displayed significant activity towards the hydrophenoxylation of internal alkynes. These addition reactions are attractive since they can be carried out without the addition of silver salts, acidic promoters, or even solvent.
References:
1. Fernandez, E. J.; Laguna, A.; Olmos, M. E. Adv. Organomet. Chem. 2005, 52, 77.
2. Liu, L. P.; Hammond, G. B. Chem. Soc. Rev. 2012, 41, 3129.
3. Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. Angew. Chem. Int. Ed. 2003, 42, 4302
4. Partyka, D. V.; Zeller, M.; Hunter, A. D.; Gray, T. G.; Angew. Chem. Int. Ed. 2006, 45, 8188.
5. Lenker, H. K.; Gray, T. G.; Stockland Jr., R. A. Dalton Trans. 2012, 41, 13274-13276.
6. Hashmi, A. S. Chem. Rev. 2007, 107, 3180-3211.
7. Li, Z.; Brouwer, C.; He, C. Chem. Rev. 2008, 108, 3239-3265.
8. Kuram, M. R.; Bhanuchandra, M.; Sahoo, A. K. J. Org. Chem. 2010, 75, 2247-2258.
9. Oonishi, Y.; Gómez-Suárez, A.; Martin, A. R.; Nolan, S. P. Angew. Chemie. 2013, 125, 9949-9953.
10. Schmidbaur, H.; Schier, A. Z. Naturforsch., B 2011, 66, 329–350.
11. Leyva, A.; Corma, A. J. Org. Chem. 2009, 74, 2067–2074.
12. Andraos, J. Org. Process Res. Dev. 2005, 9, 149–163.
Copyright © 2014 American Chemical Society