58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI151705-DNI1: Catalytic Functionalization of Carbon-Hydrogen Bonds with Alkyl Halides
Gong Chen, Ph D, Pennsylvania State University
Research Report
Key discovery I: Development of Palladium-Catalyzed Picolinamide-Directed Alkylation of Unactivated C(sp3)-H Bonds with Alkyl Iodides
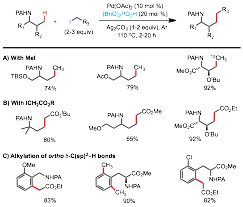
The metal-catalyzed coupling of unactivated sp3 hybridized C-H bonds with alkyl halides remains one of the most difficult challenges in the C-H functionalization field. Advances in this area could offer greatly simplified methods for the construction of C(sp3)-C(sp3) bonds from a large pool of readily accessible and economical starting materials. Despite the progress made on Pd-catalyzed C(sp2)-H alkylation reactions over the past decade, alkylation of unactivated C(sp3)-H bonds with alkyl halides has been much less advanced and protocols with synthetic relevance are even rarer. Under the support of this PRF grant, we have successfully developed a new set of readily applicable Pd-catalyzed reactions to alkylate the unactivated and remote C(sp3)-H bonds of picolinamide-protected aliphatic amines with primary alkyl iodides (Figure 1).1 The reactions require Ag2CO3 and a newly identified organic phosphate promoter (BnO)2PO2H. In particular, the use of MeI and a-iodoacetic esters provides an efficient and straightforward method to prepare high-valued N-containing products from readily available precursors.
Key discovery II: Development of Stereoselective Synthesis of b-Alkylated a-Amino Acids via Palladium-Catalyzed Alkylation of Methylene C(sp3)-H Bonds
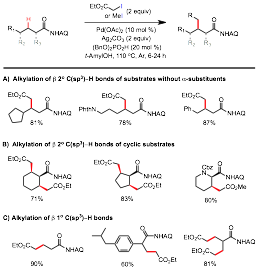
Amino acids are one of nature's most powerful and versatile building blocks for the synthesis of natural products and biomolecules. In addition to the common proteinogenic amino acids, nature uses post-translational modifications to synthesize a myriad of nonproteinogenic amino acids with diverse structures and functions. Among these modifications, alkylation at the b position of a amino acid residues, e.g. C-methyltransferase-mediated methylation, is particularly effective at modulating the conformational and biophysical properties of the parent peptide backbones. These b-alkylated amino acid units contain adjacent carbon stereogenic centers and pose significant synthetic challenge. Complementary to conventional synthesis strategies, we envisioned these molecules could be expeditiously accessed via the selective alkylation of sp3-hybridized C-H bonds on the side chains of simple amino acid precursors. Under the support of this PRF grant, we have discovered a new set of reactions based on the Pd-catalyzed alkylation of unactivated methylene C(sp3)-H bonds of aminoquinolyl aliphatic carboxamides with a-haloacetate and methyl iodide (Figure 2).2 These reactions are highly efficient, versatile, and have broad substrate scope. These reactions represent the first generally applicable method for the catalytic alkylation of unconstrained and unactivated methylene C-H bonds with high synthetic relevance. These reactions enable a streamlined strategy for the synthesis of various natural and unnatural amino acids, particularly b-alkylated a-amino acids, starting from readily available precursors in a diastereoselective manner following a straightforward template. With simple isotope-enriched reagents, they also provide a convenient and powerful solution to site-selectively incorporate isotopes into the carbon scaffolds of amino acid compounds. Applications of this C-H alkylation methodology in the synthesis of complex peptide natural products are currently under investigation.
Reference:
1. Shuyu Zhang, Gang He, William A. Nack, Yingsheng Zhao, Qiong Li, Gong Chen* J. Am. Chem. Soc. 2013, 135, 2124-2127.
2. Shu-Yu Zhang, Qiong Li, Gang He, William A. Nack, Gong Chen* J. Am. Chem. Soc. 2013, 135, 12135-12141.
Figure 1. Pd-catalyzed PA-directed Alkylation of g C(sp3)-H bonds
Figure 2. Pd-catalyzed AQ-directed Alkylation of b C(sp3)-H bonds
Copyright © 2014 American Chemical Society