58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI351217-UNI3: Functionalized, Binuclear N-Heterocyclic Carbene Ligands Bearing Phosphine Donors: Synthesis of Metal Complexes and Applications to Homogeneous Catalysis
Gregory J. Domski, Ph.D., Augustana College
During the 2012-2013 funding period my students and I explored three areas of research in order to interrogate the hypothesis laid out in the original proposal: (1) probing the effect of ortho-metalation on catalyst activity, (2) preparing novel pyridine-functionalized bisimidazolium ligand precursors, and (3) preparing bimetallic palladium(II) complexes supported by phosphine- and pyridine-functionalized bis-N-heterocyclic carbene ligands.
Probing the effect of ortho-metalation on catalyst activity
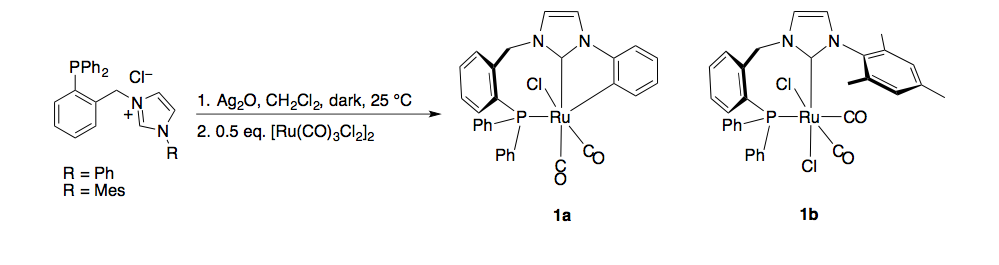
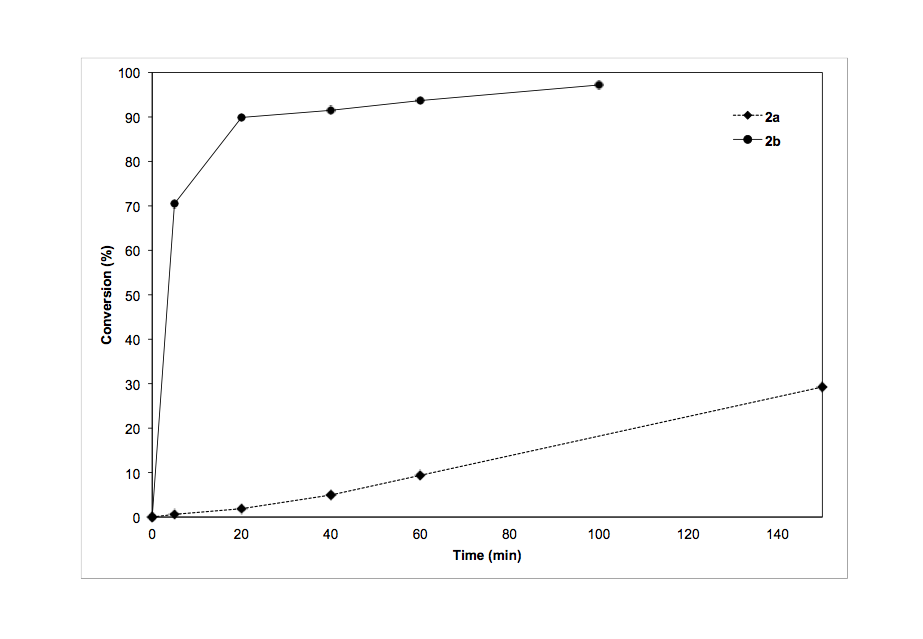
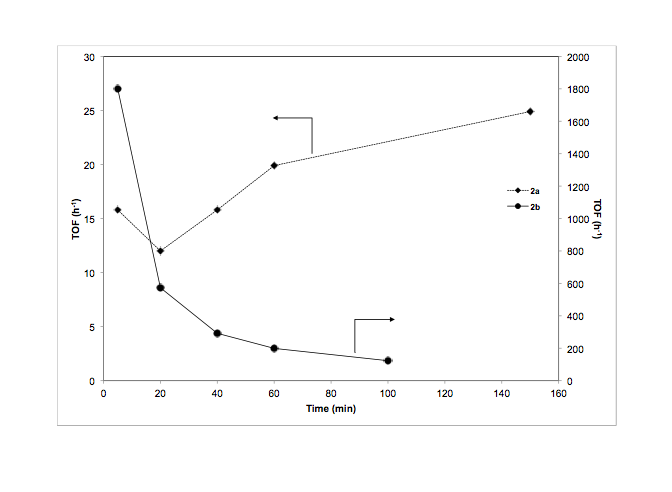
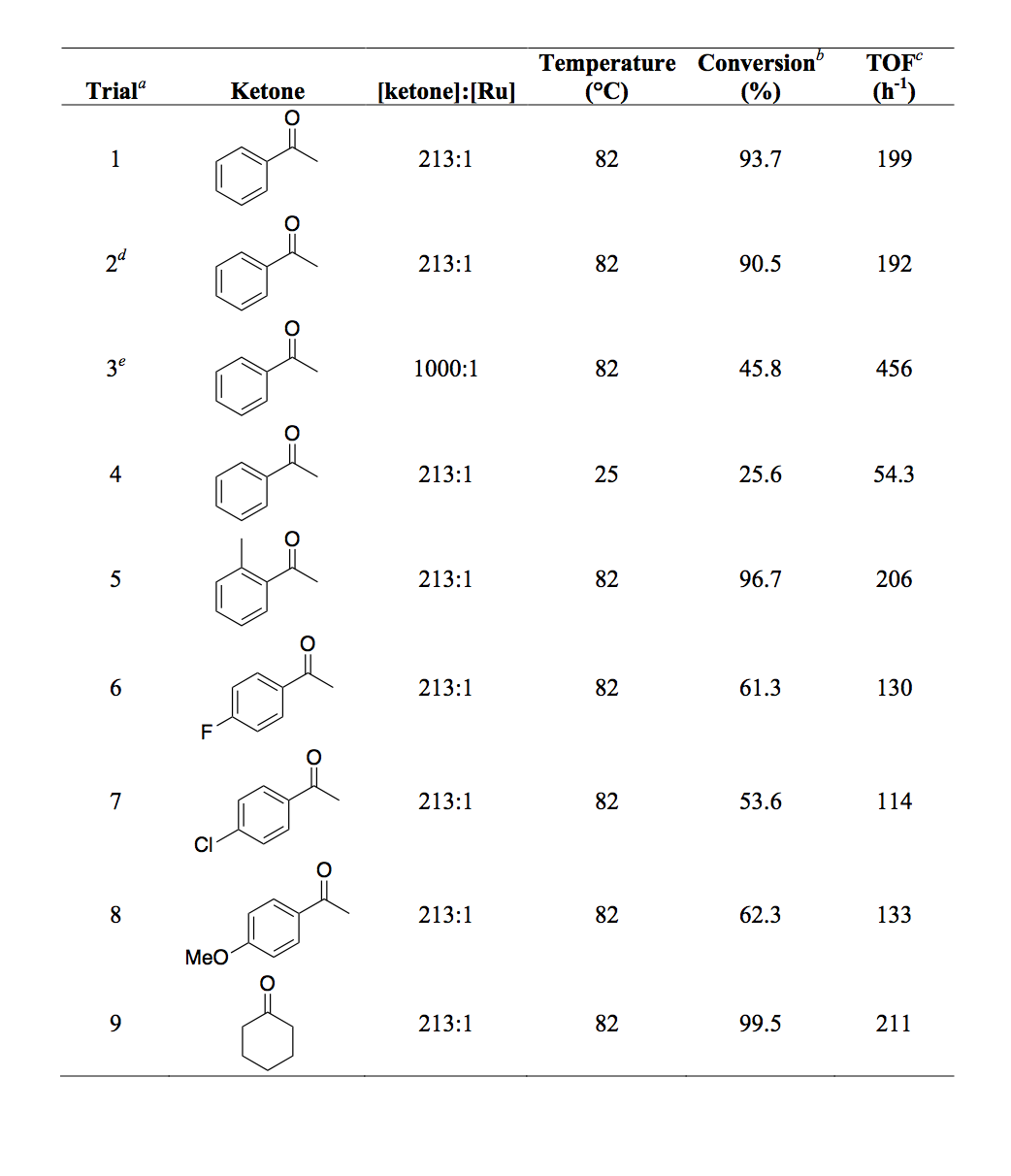
We successfully synthesized and characterized two monometallic ruthenium(II) complexes supported by phosphine-functionalized NHC ligands (figure 1, 1a and 1b). Both of these complexes were active for transfer hydrogenation of various ketones (Table 1); however catalyst 1a/KOtBu was much less active than 1b/KOtBu. We monitored the percent conversion and turn over frequency (TOF) as a function of time for both catalysts (Figures 2 and 3 respectively). From these data it appears that 1a initiates slowly as TOF increases over time. These results were recently accepted for publication in Inorganic Chemistry Communications.
Figure 1. Synthesis of monometallic ruthenium(II) complexes
Figure 2. Transfer hydrogenation of acetophenone to form 1-phenylethanol: % conversion vs. time for 1a and 1b.
Table 1. Results of catalytic trials with 1b
aGeneral conditions: 2b (16 µmol) was dissolved in 5.0 mL of dry, degassed 2-propanol along with KOtBu (99 µmol) in an N2 atmosphere. The ketone (3.4 mmol) was added to the reaction flask then the reaction mixture was heated to 82 °C with stirring. The reaction duration in each case was 60 minutes. bDetermined via gas chromatography. cmol alcohol/mole Ru/hour. dThis reaction was conducted using unpurified solvents in air. e16.0 mmol of ketone was used.
Figure 3. Transfer hydrogenation of acetophenone to form 1-phenylethanol: turn over frequency (TOF) vs. time for 1a and 1b.
Preparation of novel pyridine-functionalized bisimidazolium ligand precursors
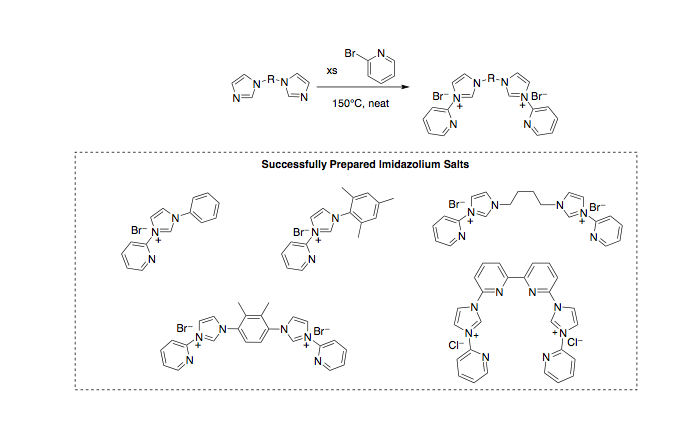
In the 2011-2012 progress report we discussed the difficulties in preparing phosphine-functionalized bisimidazlium precursors. Even though we were successful in preparing these compounds, the successful synthesis is time intensive. In order to make more rapid progress in synthesis and probing structure/function relationships we began simultaneously pursuing the synthesis of pyridine-functionalized bisimidazolium ligand precursors (Scheme 1). This route was quite successful and allowed us to quickly prepare and characterize several novel pyridine-functionalized bisimidazolium salts (Figure 4).
Scheme 1.
Preparation of bimetallic palladium(II) complexes supported by phosphine- and pyridine-functionalized bis-N-heterocyclic carbene ligands
The preparation of bimetallic ruthenium (II) complexes supported by donor-functionalized NHCs is an ongoing challenge. The ancillary carbonyl ligands make isolation and purification of these complexes difficult since six possible geometric isomers may be formed. In order to obviate the need for ancillary ligands and circumvent the complicating issue of isomers, we pursued the preparation of bimetallic palladium(II) complexes supported by phosphine- and pyridine-functionalized bis-NHC ligands (Scheme 3). From 1H NMR data it appears that the routes described are viable since the peak for the NCHN imidazolium proton is not present in the spectrum for the compounds we have prepared and other key peaks have shifted appropriately. Palladium(II) has a track record, although not as solid as ruthenium(II) of catalyzing transfer hydrogenation. Nonetheless, we will pursue catalytic transfer hydrogenation trials with the bimetallic palladium complexes and their monometallic analogues in order to investigate the hypothesis set forth in the proposal.
Copyright © 2014 American Chemical Society