58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND750813-ND7: Metallocene Catalyzed Polymerization Investigated by Hyperpolarized NMR
Christian Hilty, Dr. sc., Texas A&M University
Collaborating with other researchers within our department, we further applied DNP-NMR measurements to another polymer reaction, the ring-opening polymerization of L-lactide. Polylactide is a target for environmentally friendly plastics, since it can be degraded by microorganisms. We are investigating this polymerization reaction with a metal free catalyst. Metal free catalysts are of interest, because metal residues in polymers can be undesirable in certain cases, for example for medical applications. DNP-NMR spectra of the polymer reaction could readily be obtained, and showed buildup of the signals from the polymer species as a function of time. In particular, the signal from the ester carbon in the polymer is prominently located at 169.5 ppm. Interestingly, in addition to this expected signal, a new signal at 175.2 ppm was observed. In order to identify this peak, a correlation experiment was carried out, where the signal from the ester carbon of the monomer was selectively inverted. In this experiment, the peak at 175.2 ppm also showed negative intensity, indicating that it corresponds to this carbon atom in a product of the reaction. We are using additionl DNP-NMR experiments in order to characterize this species and for determining the reaction mechanism.
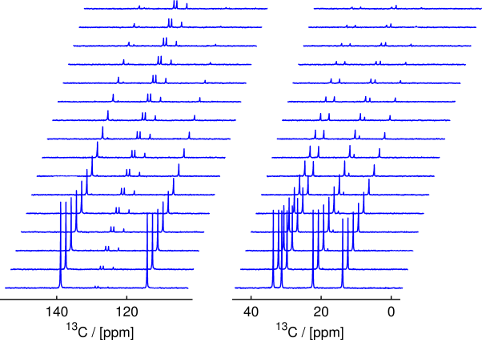
Figure 1: Stacked plot of time series of spectra acquired from a hyperpolarized sample of 1-hexene using small-flip angle excitation (without reaction; acquired at time intervals of 400 ms). The two panels show two different spectral regions.
Copyright © 2014 American Chemical Society











