58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI451896-UNI4: Gas Phase Ion Dissociation Dynamics through Photoionization Mass Spectrometry
James P. Kercher, PhD, Hiram College
The dissociation dynamics of energy-selected ions of a variety of compounds have been investigated by Photoelectron Photoion Coincidence (PEPICO) Spectroscopy through the support of the ACS-PRF 51896UNI4 award. The award has supported several student research projects over the first year. Four chemistry majors were able to conduct experiments at the Swiss Light Source (SLS) in Villigen, Switzerland and two students were supported for 10 week summer research experiences. Our initial proposal focused on a series of germanium chloride ions, Ge(CH3)nCl(3-n)+, and ethyl chloride ions. The data on the methyl germanium series has been recorded, but has proven to be difficult to analyze due to the non-statistical nature of the dissociation. The data on ethyl chloride has also been recorded using a temperature controlled TPEPICO apparatus, but will be collected again on the double imagining PEPICO at the SLS in the near future. While working on these compounds from the initial proposal, we have studied and analyzed several other compounds including Fe(CO)5, Cr(CO)6, acetone-h6, acetone-d6, acetaldehyde, anisole, and anisole-d8. The results of Fe(CO)5have been published in J. Phys. Chem. A., and the acetone-h6 and acetone-d6 results are being prepared for submission. The work on these compounds is described herein.
Research Progress
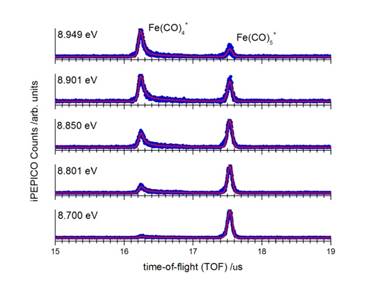
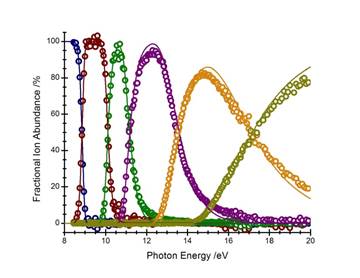
Ironcarbonyl, Fe(CO)5 : The dissociation dynamics of internal energy selected iron carbonyl cations, Fe(CO)5+, have been investigated using the imaging photoelectron photoion coincidence (iPEPICO) spectrometer at the Swiss Light Source. The molecular ion loses all five carbonyl ligands in sequential dissociations in the 8.5–20 eV energy range. The parent ion is metastable at the onset of the first dissociation reaction on the time scale of the experiment. The asymmetric and broad daughter ion time-of-flight distributions shown in figure 1 indicate parent ion lifetimes in the microsecond range, and are used to obtain an experimental rate curve. Further carbonyl losses were found to be fast at threshold. The fractional parent and daughter ion abundances as a function of the initial internal energy of the parent ion, i.e. breakdown diagram (figure 2), as well as the dissociation rates for the first CO loss were modeled using the statistical RRKM theory for unimolecular reactions. The excess energy redistribution was also taken into account in a statistical framework.
The 0K dissociative photoionization thresholds for the five carbonyl-loss channels were determined for the processes leading to Fe(CO)4+, Fe(CO)3+, Fe(CO)2+, Fe(CO)+, and Fe+, respectively. The iron cation thermochemistry is well known and these onsets connect the iron ion to the other fragment ions as well as to the gas phase neutral Fe(CO)5. The results of this work have been published in J. Phys. Chem. A. (see J. Phys. Chem. A. 117(22) 4556-4563 (2013).
Acetone and Acetone-d6: Many ionic dissociation reactions proceed to products with no barrier. However, some reactions that lose molecular fragments, involve rearrangements of the skeleton structure prior to the loss of the molecular fragment. An example is the loss of methane from acetone to yield the ketene ion:
CH3COCH3 → CH2CO+ + CH4 (1)
This reaction, which is the lowest energy dissociation channel for acetone ions, involves a transfer of an H atom from one methyl group to the other methyl group. Other examples include HCl loss from C2H5Cl+. A recently published paper on the H atom loss from formic acid based on experimental work at the VUV beamline is another example. The experimental results clearly showed that, although this reaction involves just an H atom loss, the reaction proceeded via a substantial barrier. High level calculations confirmed this. In that case, calculated rates using the Eckart tunneling barrier predicted rate constants quite close to the experimental ones but this reaction was rather an ideal one because a one dimensional model was adequate. When dealing with an H atom transfer in acetone, several normal vibrational modes of the ion must be involved in this isomerization. This reaction thus presents an excellent test of the adequacy of a 1-D model is for such reactions. The advantage of the Eckart barrier model is its simplicity.
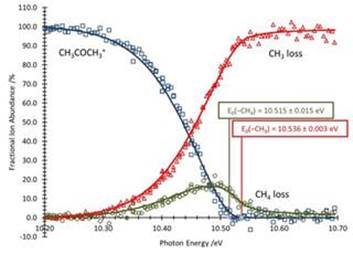
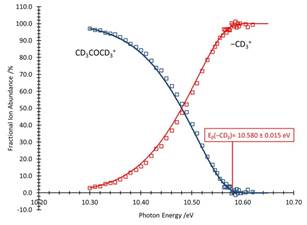
The breakdown curve for acetone and acetone-d6 are given in figures 3 and 4, respectively. Immediately evident is that the methane loss reaction is not competitive in the deuterated molecule; only the CD3loss channel is observed. This is an indication that the reaction proceeds solely by tunneling.
We are currently modeling these reactions and completing high level calculations to determine the dissociation thresholds and barrier heights. The results of this work will be submitted for publication shortly.
Conclusion
We have recorded data on several molecules using imaging and threshold PEPICO techniques. We are currently analyzing data for our initial proposal while working on other interesting data sets. Four Hiram College undergraduates have conducted experiments at the SLS through two successful proposals for experimental beamtime. A total of six Hiram College undergraduates have contributed to these studies over the first year of the grant. Three of these students are lead authors on the iron carbonyl paper. Three others will be authors on the acetone paper. We will continue to submit proposals to the SLS over the next funding year of to continue this research.
Broader Impact:
The generous funding from the American Chemical Society Petroleum Research Fund has been an integral part in establishing physical chemistry as an exciting area of study at Hiram College. Students have been introduced to a variety of techniques including time-of-flight mass spectrometry, computational chemistry, and statistical modeling techniques. Incorporation of this research into the classroom has unified the teaching of the physical chemistry curriculum.
The ACS PRF grant has had a very important impact on my career as an educator as well. The financial support has allowed me to incorporate a computational software package into the curriculum, develop experiments for physical chemistry labs which combine synthesis, investigation using the PEPICO mass spectrometer and support via computational methods. I look forward to working closely with the American Chemical Society Petroleum Research Fund in the future.
Copyright © 2014 American Chemical Society