58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND252142-ND2: A Potential Fungal Contribution to the Selective Preservation of Long-chain Hydrocarbon Functionality in Soils and Sediments
Neal E. Blair, Ph.D., Northwestern University
To determine if soil fungi can produce recalcitrant aliphatic material, we initiated degradation experiments of a common temperate soil saprotroph, Fusarium avenceum, in laboratory soil microcosms and in the field. To perform these experiments we developed a procedure employing 100μm mesh stainless steel packets to retain the fungal material. The stainless steel mesh offers the advantage over plastic litterbag materials in that mesh sizes are smaller (and thus can retain the fungal necromass) and we are able to obtain FTIR spectra of the contents of the packets without disassembly and interference from the mesh material. This allows us to investigate small scale spatial variability in chemistry. Samples were analyzed by both FTIR and tetramethyl ammonium hydroxide (TMAH)-thermochemolysis GC-MS.
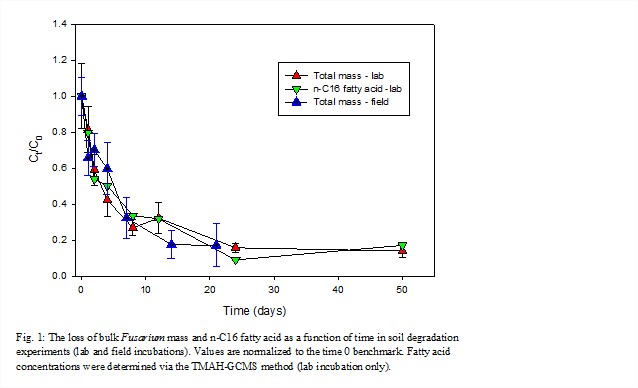
Fungal material was rapidly lost as indicated by bulk mass and major biochemical constituents, such as the dominant lipid the n-C16:0 fatty acid (Fig. 1). There was no significant difference between laboratory and our initial field results.
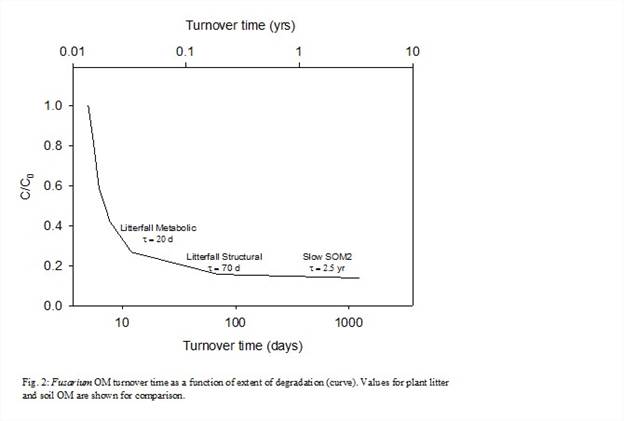
The kinetic data indicate that multiple components of varying reactivity exist (Fig. 2). Initial turnover times (τ ~ 5 days) are consistent with previous studies of ectomycorrhizal necromass. As reactive fractions are removed, the bulk turnover time evolves through values equivalent to the metabolic (reactive) and structural (cellulose/lignin) components of plant litter, as well as the slowly degrading fraction of soil organic matter.
Approximately 70% of the Fusarium OM had a turnover time of <10 days whereas >10% had a potential turnover time of 3 yrs or longer (Fig. 2). The experiments performed thus far demonstrate that the Fusarium necromass is composed of biochemical fractions with different reactivities that span those of plant litter and possibly slowly reacting soil OM.
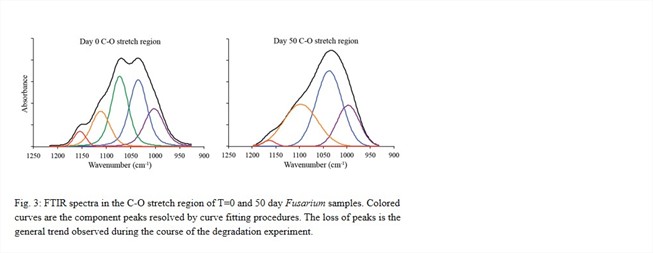
The identity of the most recalcitrant fraction of Fusarium is unknown, however both TMAH and FTIR analyses provide initial characterization. FTIR microspectroscopy of a mesh-bound sample after 50 days of incubation indicates some infiltration by clays into the packet but most of the fungal tissue seems to be unassociated with inorganic minerals. Protection via aggregation with clays thus does not seem to be an important factor in this experiment. FTIR analysis also reveals changes in organic composition over time. The reduction in the absorbance of a C-O stretch centered at 1073 cm-1 is likely due to the loss of a dominant carbohydrate, such a β-glucan (Fig. 3). Additional C-O containing components as well as amide functionality persists to 50 days, though with visible changes in their relative abundance. One hypothesis is that this material is chitin or chitosan.
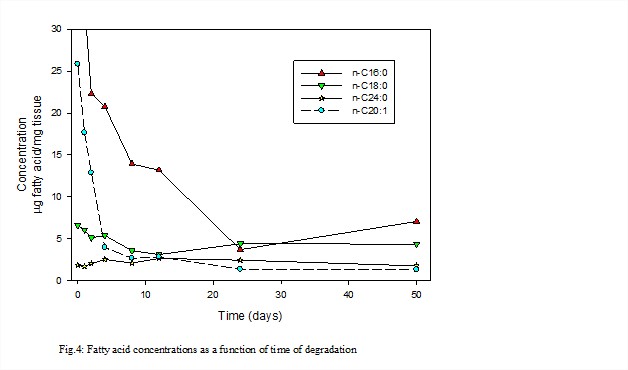
Lipids display widely variant behaviors. Whereas the n-C16:0 and n-20:1 fatty acids are lost rapidly relative to bulk fungal mass, the n-C18:0 and n-24:0 fatty acids exhibit modest changes in concentration (Fig. 4). This suggests that the C16 and C20 acids are associated primarily with a reactive fraction, such as a glycolipid component, and the C18 and C24 compounds are preferentially bound in a more recalcitrant fraction. It also clearly illustrates that compound structure is not the sole determinant of reactivity. Protective matrices, including those biochemical, can have a disproportionate effect on reactivity. Future research will be directed to determining if other fungal species produce recalcitrant biochemical fractions, and identifying the composition of the protective matrix and fatty acid binding method.
This research project has led to new directions for my research group in multiple dimensions. This is the first research that we have done involving soil fungi and macromolecular biochemistry. This is a significant departure from our traditional marine sediment C-isotope work and provides a new addition to our analytical and experimental approach toolboxes. Given the acknowledged ubiquity of soil fungi and their importance to the global soil C-cycle, the observations made thus far concerning their ability to sequester fatty acids may have a major impact on the interpretation of the organic geochemical record.
Copyright © 2014 American Chemical Society