58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI1052323-UNI10: Morphology-Controllable Synthesis and Characterization of Low-Temperature Active Rare-Earth Oxide Nanocatalysts
Ruigang Wang, Youngstown State University
Synthesis of shape-controlled CeO2 nanocrystals
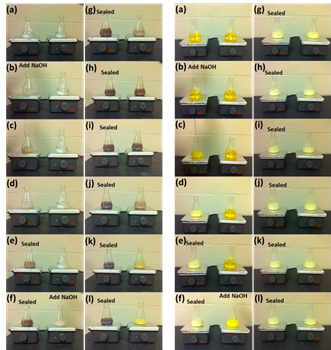
The effect of varying the starting materials and experimental conditions on the shape control of CeO2 nanocrystals using hydrothermal method was investigated, including using different cerium precursors (Ce3+: Ce(NO3)3 and Ce4+: (NH4)2Ce(NO3)6) etc. It was found that the formation of Ce(OH)3 nuclei is a key step to growth of different morphological CeO2 nanocrystals. Figure 1 shows the results in two comparison experiments using different cerium precursors 0.1M Ce(NO3)3 and 0.1M (NH4)2Ce(NO3)6, with and without the stirring treatment of the solution mixture before hydrothermal reaction. When using trivalent cerium (III) precursor (Ce(NO3)3), the expected Ce(OH)3 precipitate was unstable in air at even room temperature, and the final product showed a yellow color originated from lilac after 20 min stirring. This indicates the occurrence of oxidation of the sample as only CeO2 or Ce(OH)4 with tetravalent Ce4+ possess yellow color. When using ammonium cerium (IV) nitrate precursor ((NH4)2Ce(NO3)6) as the starting cerium precursor, no continuous color change was observed. The color change for the unsealed Ce(NO3)3 sample clearly indicates an occurrence of oxidation during the stirring. A possible explanation for the color change when using trivalent cerium precursor Ce(NO3)3, could be as follows, involving a precipitation step (I) of the cerium precursor, followed by a dehydration/oxidation step (II):
(i) Ce(NO3)3 (ii) Ce(NH4)2(NO3)6
Figure 1. The color change of the precipitate suspension for the sealed and unsealed samples with continuous stirring for a mixture of 6 M NaOH solution with different cerium precursors ((i) Ce(NO3)3, (ii) Ce(NH4)2(NO3)6). The left flask was sealed and the right one was not sealed after NaOH addition during the 20 min stirring.
The actual occurrence of these two reactions depends on the availability of the dissolved oxygen in the solution and oxygen at the air/liquid interface. Ce(OH)3 nuclei form as soon as Ce3+ ions are mixed with NaOH solution. Ce(OH)3 is not stable in air, and thus when vigorously stirring the solution in air, Ce(OH)3 can react with oxygen and then become oxidized to CeO2. The role of stirring may relate to the kinetics of oxygen/Ce(OH)3 gas-solid interaction, thus favoring the formation of CeO2 by introduction of stirring. For the unsealed sample, both steps I and II can proceed completely resulting in the formation of yellow CeO2, while for the sealed sample, the availability of oxygen was limited, so only a relatively small quantity of Ce(OH)3 can be transformed to CeO2, leading to no color change after about 6 min during stirring.
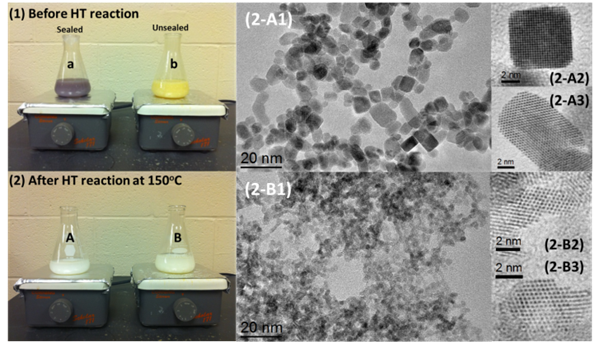
Figure 2 compares the color difference of the suspension solutions between the sealed and unsealed samples using Ce(NO3)3 before and after the hydrothermal reactions at 150oC for 48 hrs. After the hydrothermal reactions, both of the samples show white color, but high resolution TEM (HRTEM) images show that the nanoparticles for the unsealed sample have an irregular shape with a size of 3~4 nm, while most of the nanoparticles for the sealed sample display nanocubes/nanocuboids with a size of 6~10 nm. The sealed sample using Ce(NO3)3presents the best crystallinity and largest particle size.
Figure 2. Effect of oxidation of Ce(OH)3on the shape/size control of ceria nanoparticles. Both of the samples were stirred for 20 min. Sample A was sealed during the stirring, while sample B was not. HT: hydrothermal.
The observation above demonstrated that the formation (and/or shape/size) of Ce(OH)3 crystal seeds play a critical role in synthesizing shape/size-controlled CeO2 nanocrystals. Based on this strategy, we prepared CeO2 nanocrystals of different morphology at different temperatures and dwell times, in which the Ce(NO3)3/ NaOH suspension solutions were not stirred and quickly transferred to autoclaves for the hydrothermal reactions at different temperatures and dwell times. Figure 3 shows representative TEM images of CeO2 nanocrystals with controlled morphology. The monodisperse CeO2 nanocrystals with different shapes and sizes including nanosheets, nanorods, nanocubes, and nanocuboids can be obtained by the control of reaction temperature and dwell time. In general, the CeO2 nanocrystals seemed to prefer the morphology of nanosheets and nanorods at low-temperature (130oC) hydrothermal conditions, while at higher temperature the nanocube-like or irregular shapes were observed.
Figure 3. Some typical morphologies of CeO2nanoparticles prepared by a facile hydrothermal method at different temperatures and dwell time: (a): 70oC; (b): 110oC; (c): 130oC; (d): 210oC.
Reducibility (or CO consumption) of CeO2 with different shapes
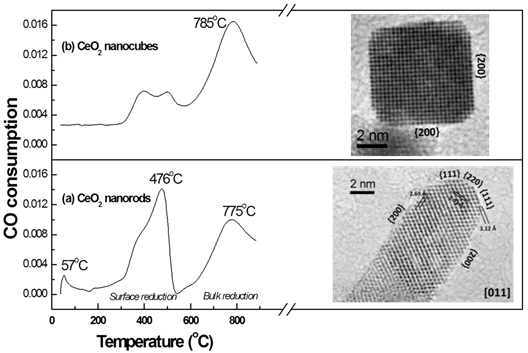
We have used CO (carbon monoxide) temperature programmed reduction (TPR) to measure the CO consumption (2CeO2+CO →Ce2O3+CO2) during the reduction of CeO2. Figure 4 shows the CO-TPR profiles and high resolution TEM images for CeO2 nanorods and nanocubes, which shows a significantly improved low-temperature surface reduction of CeO2 nanorods. The corresponding TEM shape and atomic-level surface structural study of these nanoparticles are showed in Figure 4. We will use CO-TPR to characterize redox properties and CO consumption of CeO2during reduction, which are directly related to catalytic activity.
Figure 4. CO-TPR profiles and high resolution TEM images of CeO2nanorods and nanocubes.
Impact on PI and the students
Since beginning at YSU in 2010, PI has initiated a research program in materials chemistry. This ACS PRF grant strongly helped PI recruit several student researchers in the group and support PI¡&hibar;s research during the summer 2012. So far, this funding also leaded to publications with graduate/undergraduate co-authors in RSC advances, Journal of Materials Chemistry and Advanced Materials Research. In PI¡&hibar;s laboratory, students plan and set up hydrothermal reaction, use characterization techniques including PXRD, SEM, TGA, and IR/Raman, read relevant literature, and write about and present their work. Three undergraduates and two high school students have worked in PI¡&hibar;s lab for a semester or more. One graduate student started to work on the current project from Fall semester 2013. In addition to three students have been coauthors on seven presentations PI has made at regional and national meetings.
Future activities
We are currently finishing up the shape-catalytic activity correlation experiments. We will be investigating the atomic level structure and chemistry of the low-temperature active cerium based oxide nanocrystals.
Copyright © 2014 American Chemical Society