58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI551723-DNI5: Mechanism of Oxidative Dehydrogenation of Alkane on Supported Vanadium Oxide
Zhenrong Zhang, PhD, Baylor University
Technical Progress Report
The research objective of this proposal is to understand how the local structure influences the oxidative hydrogenation of alkane related reactions on TiO2 supported VOx model oxide nanocatalysts. In the first year (1/01/2012 — 8/31/2013), we have worked on two correlated projects in parallel. One is the surface reaction of ketone and diols on rutile TiO2(110). Variable temperature scanning tunneling microscopy (VT-STM) was used to ascertain the site-specific elemental reaction steps (adsorption, dissociation and rotation). The other project is the atomic structure study of anatase TiO2(001) thin films. The goal is to adapt high quality anatase films ex situ for the molecular-level surface reaction mechanism study.
Project I: Surface Reactions on Rutile TiO2(110)
· Surface Reactions of Acetone on TiO2(110)
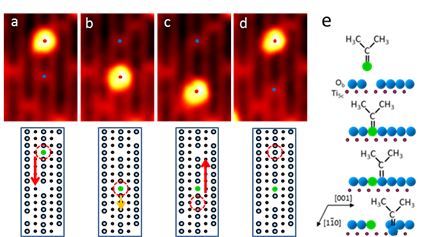
Point defects are of fundamental importance for the chemistry and photochemistry of metal oxides. We have studied the dynamic relationship between acetone and bridge-bonded oxygen (Ob) vacancy (VO) defect sites on the TiO2(110)-1 x 1 surface using scanning tunneling microscopy (STM) and density function theory (DFT) calculations. We report an adsorbate-assisted VO diffusion mechanism. The STM images taken at 300 K show that acetone preferably adsorbs on the VO site (Figure 1a) and is mobile. The sequential isothermal STM images directly show that the mobile acetone effectively migrates the position of VO by a combination of two acetone diffusion channels: one is the diffusion along the Ob row and moving as an alkyl group (Figure 1b), which heals the initial VO; another is the diffusion from Ob row to five coordinated Ti4+ row and then moving along the Ti4+ row as an acetone (Figure 1a, 1c), which leaves a VO behind. The calculated acetone diffusion barriers for the two channels are comparable and agree with experimental results.
Figure 1. STM images recorded as a function of time (t = 0, 12, 18, 21 min) on the same area of acetone pre-adsorbed TiO2(110). The corresponding ball models illustrate the diffusion of VO mediated by acetone diffusion at 300 K.
· Reactions of Acetone with Oxygen Adatoms on TiO2(110)
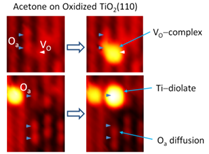
Understanding the interaction of O2 with ketones on metal oxide surfaces is important for the oxidation of organic molecules. The consecutive reaction steps of acetone molecules with oxygen adatoms (Oa's) on partially oxidized TiO2(110) surfaces have been studied using high-resolution STM at 300 K. The sequential isothermal STM images reveal two types of acetone-Oa species as a result of reactions of acetone with an oxygen adatom and oxygen vacancy (Figure 2). One species is the Ti5c-bound acetone-Oa diolate formed from Ti5c-bound acetone reacting with Oa. The diolate is mobile at 300 K and can assist the diffusion of surface Oa by exchanging the acetone oxygen with the Oa. The second acetone-Oa species is VO-bound acetone-Oa complex formed from a VO-bound acetone reacting with an Oa located on the neighboring Ti row. The VO-bound complex is stationary at 300 K. This species has not been reported previously.
Figure 2. STM images revealing the interaction of acetone with oxygen adatoms (Oa) and oxygen vacancies (VO) on oxidized TiO2(110).
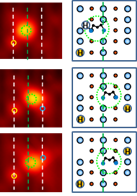
We have employed
variable-temperature STM and dispersion-corrected DFT to study the interactions
of 1,2-ethylene and 1,3-propylene glycols with reduced TiO2(110)
surfaces. We found that at 300 K, both glycols dissociate via O-H bond scission
of one of the OH groups on oxygen vacancy defects forming pairs of monoalkoxide
(Ob-(CH2)n-OH, where n = 2 or 3) and
bridge-bonded bonded hydroxyl (HOb) species. The Ob-(CH2)n-OH
species are observed to rotate around their Ob anchor, switching the
position of the OH between the two adjacent Ti5c rows (Figure 3).
The rotating species are found to assist cross-Ob row HOb
hydrogen transfer. The OH group of the monoalkoxide species is further observed
to dissociate forming a bidentate type dioxo (Ob-(CH2)n- Figure
3. STM images
showing the rotation of monoalkoxide
resulted from dissociation of 1,3-propylene glycols TiO2(110).
Project II:
Surface Reactions on anatase TiO2(001) ·
Anatase TiO2(001) thin films The anatase
polymorph of TiO2 appears to be an even more potent catalyst than
rutile. Understanding
the structure of well-defined anatase TiO2 surfaces is critical for
deciphering site-specific thermal and photo- reaction mechanisms on anatase TiO2.
We have successfully resolved the surface structure of anatase TiO2(001)
thin films
grown by oxygen plasma assisted molecular beam epitaxy. These
well-defined surfaces are ideal to correlate the structure with the
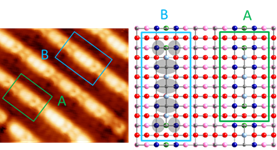
activity at the molecular level. High-resolution STM images taken from the same
area at different bias voltages show that the structure of the bright row is
originated from combinations of two basic atomic building blocks (Figure 4). We
propose a modified added molecule model for the anatase TiO2 (001)
surface structure.
Figure 4. STM images showing that the atomic
structure of the bright row of anatase TiO2(001) (1×4) epitaxial thin films is
originated from two atomic building blocks.
Impact of the Research
on My Career and the Students This PRF new investigators
grant enables me to establish an active research program at Baylor University
in the critical third year of my tenure-track. The results from the supported
research are published in four peer-reviewed publications in prestigious
international journals. My group presented six oral/poster presentations
at five international, national, and regional professional conferences. The
support and publications build the solid foundation for my federal funding endeavors.
Two graduate
students were partially supported by this grant. This support enables one
student (Yaobiao Xia) conduct the major part of his thesis work. I am proud
that he won a best poster prize at Southwest Catalysis Society 2012 Spring
Symposium. He will graduate next year. Four undergraduate students are involved
in the projects. Two of them (Amir Ali and Blake Birmingham) won the
Outstanding Poster Presentations at university-wide Undergraduate Research
Scholars Week.
![]() ) species and an
additional HOb. The reversible interconversion between the mono- and
di-oxo species clearly shows the attainment of a dynamic equilibrium between
these conjugate acid/base pairs.
) species and an
additional HOb. The reversible interconversion between the mono- and
di-oxo species clearly shows the attainment of a dynamic equilibrium between
these conjugate acid/base pairs.
Copyright © 2014 American Chemical Society