58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI451292-UNI4: Using the Photo-Fries Reaction as a Photochemical Probe to Quantify the Cage Effects of Ionic Liquids
Amy E. Keirstead, PhD, University of New England
Introduction. Room-temperature ionic liquids (ILs) are molten salts that are liquids at or below room temperature and have recently attracted considerable attention as environmentally friendly alternatives to conventional (molecular) solvents. Ionic liquids exhibit extremely low volatility, high ionic conductivity, high thermal and chemical stability, and are generally nonflammable and air-stable. The versatility of ionic liquids and their robustness under extreme conditions make them ideal for a wide variety of applications, including solvents for synthesis and catalysis, host materials for molecular electronic and optoelectronic devices, electrolytes in dye-sensitized solar cells (DSSC) and for use in CO2 sequestration. Despite the rising popularity of ILs, the physicochemical properties of these interesting materials have yet to be fully characterized. In this project, the cage effect of ILs is under investigation; the cage effect is a measure of restriction placed on solute molecules by the solvent cage, and describes how effectively the species can escape the solvent cage. Ionic liquids could have large cage effects due to their viscous and ionic nature that could result in strong electrostatic interactions with guest species. These cage effects could influence product distributions and/or catalytic activity in IL-solute systems; likewise, the efficiency of the iodide/triiodide redox couple in the IL-containing DSSCs could be dramatically reduced if the IL has a large cage effect. An enhanced understanding of IL cage effects obtained via this study can be combined with other known properties of ILs (such as viscosity and polarity) to allow for the tailoring of ILs to specific applications, including the construction of more efficient DSSCs and the use of green "designer solvents" for chemical processes.
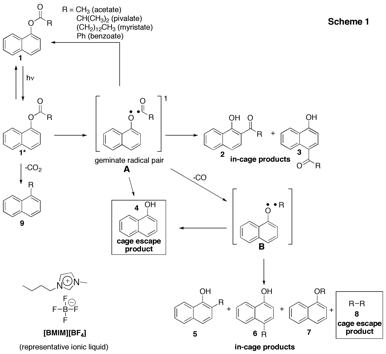
Goals and progress made. The photo-Fries reaction is the photochemical probe reaction used to report on the cage effects of ILs. In this reaction (Scheme 1), the photoexcitation of an aryl ester results in homolytic cleavage of the ester C-O bond to form a radical pair located within the solvent cage. A number of reaction pathways are available to these radicals, where the products can either be classified as in-cage (IC) or cage-escape (CE) products. Gas chromatography-mass spectrometry (GC-MS) is used to identify and quantify the photoproducts and determine the ratio of the yields of all IC and CE products.
The primary goals for the first reporting period were (i) to complete remaining groundwork and address reviewer concerns and (ii) to use this foundation towards generating higher-quality replicates of the preliminary data before (iii) extending to other ester-IL systems to examine how the cage effect is influenced by IL properties (viscosity, polarity) and those of the ester probe (steric/electronic factors). Though some progress was made, replication of these experiments in the current reporting period using a new, state-of-the-art GC-MS (acquired in Fall 2012 through an NSF MRI award) revealed that further modifications to the experimental protocol were required before reliable, meaningful data could be collected.
The first modification involved determining the ideal concentration and irradiation time to maximize photoconversion while avoiding unwanted secondary photochemistry. The more sensitive GC-MS detected small quantities of photoproducts that were previously thought to be absent, necessitating a revised protocol. The second modification entailed the procedure for extracting the photoproducts from the ionic liquid. Although the previous protocol was thought to not allow any unintentional photolysis from stray light, the more sensitive GC-MS indicated that some photolysis was taking place during the extraction process. Additional precautions to shield the samples from light were taken and proven to be effective in avoiding unwanted photolysis. Further, the extraction process was slightly modified to achieve total mass balance.
Once these "groundwork" experiments had been completed and incorporated into the standard experimental protocol, new, more reliable data was generated to compare to the preliminary results. This data for 1-naphthyl acetate in hexanes as well as two ILs, [BMIM][BF4], and [BMIM][PF6] is given in Table 1. These results corroborate the preliminary data but are thought to be more reliable due to the higher sensitivity of the instrument and improved experimental protocol. Notably, while both in-cage and cage escape products were obtained upon photolysis of 1-naphthyl acetate in hexanes, no cage escape product was observed upon carrying out the same reaction in either of the ionic liquids studied. These results suggest that the ILs exert a very strong cage effect on the nascent geminate radical pair (Scheme 1). An additional observation is the total absence of the methylnaphthalene photoproduct in the ILs compared to its formation in hexanes. Previous reports have indicated that rigid media such as polymers and films prohibit the conformation required for this excited state decarboxylation, so its absence in ILs, which are fluid media, is both notable and intriguing.
Future work. Having completed all "groundwork" and with new sensitive and reliable instrumentation, experiments to study additional naphthyl esters in a variety of ionic liquid media of varying viscosities (as indicated in point (iii), above) are ongoing. Although the data from the two ILs is not dramatically different, less viscous ILs might show more cage escape products. Esters with larger alkyl chains will also allow for the identification of photoproduct 8, a key "marker" in this study (Scheme 1).
Greater impacts. UNE is a primarily undergraduate institution with a reputation of providing high quality research experiences for its students. Tyler Rioux worked on this project during summer 2013 and is now considering a post-graduate research path (Ph.D. program). His leadership, critical thinking and scientific skills have improved since working on this project and he is currently helping to train new students. Mr. Rioux has presented his work at college symposia and will travel with Dr. Keirstead to Montreal, Canada to present his results at an upcoming meeting. This award has helped Dr. Keirstead outfit her research lab and to fund undergraduate students as well as student travel. Further, this ACS PRF award was instrumental in gaining support from the NSF MRI program to purchase the state-of-the-art GC-MS used in this work.
Copyright © 2014 American Chemical Society