58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1052146-DNI10: Synthesis of Metastable Solid State Materials Using Organometallic Reagents
Tyrel M. McQueen, PhD, Johns Hopkins University
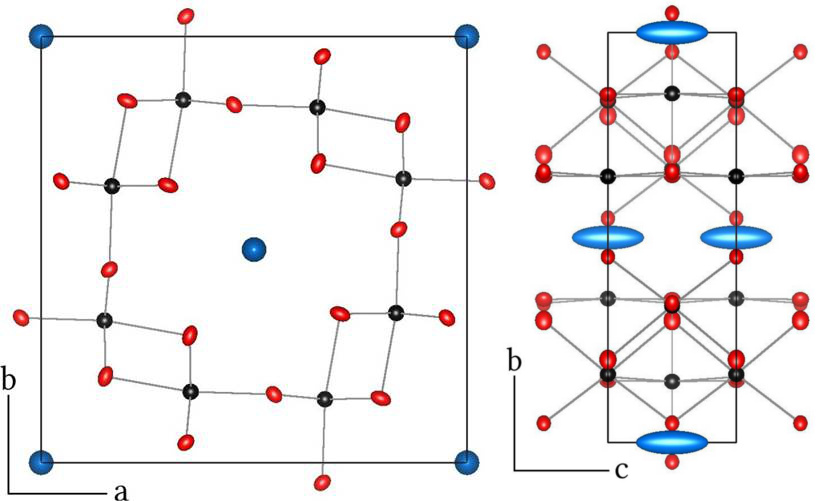
Figure 1: Structure at T = 110 K of the only known 5d transition metal Hollandite, KIr4O8 (Ir = black, O = red, K = blue). The elongated atomic displacement parameter of potassium along the channel direction reflects the mobility of potassium in the structure, allowing for chemical deintercalation near room temperature.
In work with Hollandite-type materials, we verified our initial hypothesis that it is possible to control the electron count in the transition metal framework by low temperature reaction methods. After developing a robust high temperature synthesis of the parent compound KIr4O8 (Figure 1), which is non-trivial due to the volatility of potassium and iridium, we were able to systematically control the potassium content through oxidation in acetonitrile at room temperature, as demonstrated by the systematic change in lattice parameters (Figure 2). In all cases, we found, unexpectedly, that the reactions reached completion in times short enough to preclude a direct kinetic analysis.
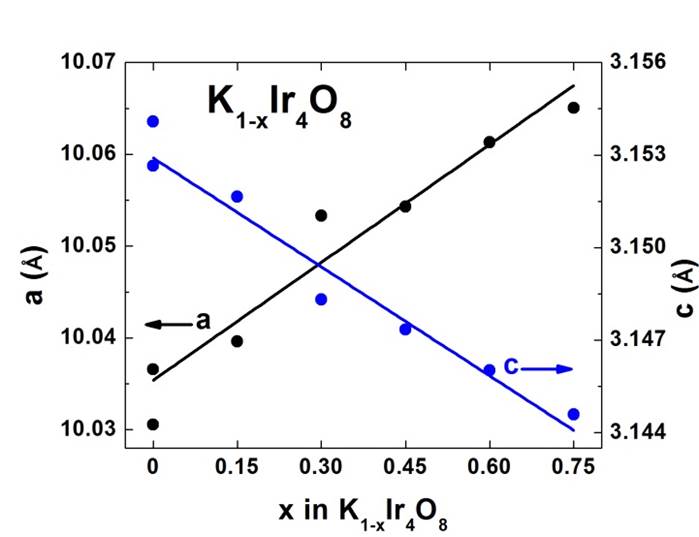
Figure 2: Systematic control of potassium content in the Hollandite KIr4O8 through reactions at room temperature in acetonitrile.
To understand the mechanistic origin of the surprisingly rapid deintercalation of potassium, density functional theory calculations were performed to study the energy landscape of potassium ion motion in KIr4O8. The energy landscape along the channel is very shallow (Figure 3). Fitting a harmonic potential (black solid line) to the calculated values and solving for the vibrational energy levels (green lines) shows that multiple excited vibrational states, corresponding to significant (0.3 A) displacements of the potassium ions, are thermally accessible even at T = 110 K. This is consistent with the experimentally observed elongated atomic displacement parameter of potassium. It also explains the high degree of reactivity and fast kinetics at room temperature: diffusion of potassium ions to the surface of the particles is the rate limiting step in the deintercalation chemistry. The shallow potential indicates a significant ability for potassium to rattle in the structure and is consistent with a low barrier to potassium ion diffusion.
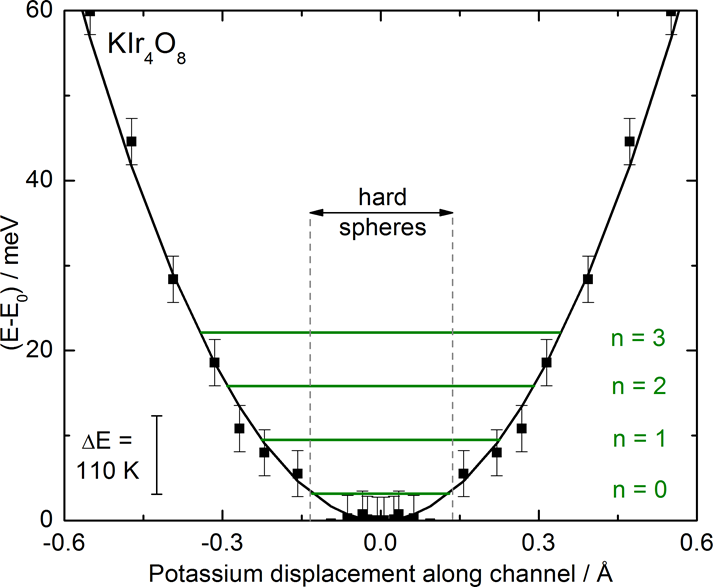
Figure 3: Calculated energy landscape for potassium ion motion in the Hollandite KIr4O8. These results explain the high reactivity at room temperature.
We have come up with a simple structural model, based on a competition between local charge neutrality (bond valence) and electron-electron repulsion (atom size) that explains this feature in KIr4O8, which is common to many Hollandite materials. We are now exploring whether it is possible to use this insight to predict what other low temperature solid state reactions will be possible. For example, it is desirable to prepare these Hollandites with a formal iridium oxidation state closer to 3+ than what is possible with potassium in the channels (3.75+). We have found that subsequent insertion of barium using a barium-benzophenone adduct in tetrahydrofuran, which should allow accessing formal iridium oxidation states closer to 3.5+, is possible (not shown), and will be investigated in detail in the coming year.
Support from the PRF has enabled our young group to generate initial results worthy of publication. This early research is expected to make future grant applications more competitive; we have had significant recent success in obtaining public and private funds, including an NSF-CAREER award and a Packard fellowship for science and engineering. In addition, students have been exposed to synthetic solid state chemistry techniques and have gained familiarity with diffraction methods and electrical and magnetic property measurements, all of which are important skill sets for their future careers. Work in progress includes several avenues of exploration: (1) following up on the insertion chemistry of barium (and other alkali earths) into the Hollandite structure; (2) continued development of spectroscopic methods to probe the rate limiting steps of solid state intercalation and deintercalation reactions; and (3) applying the insight of the competition of local energetic factors to selectively remove alkali or alkali earth ions from a single structure (dependent on reaction conditions, so we have complete chemical control), and replacing the ions using organometallic insertion reagents.
Copyright © 2014 American Chemical Society











