58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR1050797-UR10: Synthesis of Novel Polycyclic Aromatic Hydrocarbons Derived from Benzotriphenylene
Kenneth E. Maly, Ph.D., Wilfrid Laurier University
Polycyclic aromatic hydrocarbons (PAHs) have emerged as a class of organic semiconductors that have potential utility in photovoltaic solar cells, field effect transistors, and light emitting diodes. The semiconducting properties of these materials depend on both molecular properties (such as HOMO and LUMO energy levels) and effective π-overlap between neighboring molecules. Therefore, understanding and controlling molecular organization in PAHs is important for the design of new semiconducting materials.
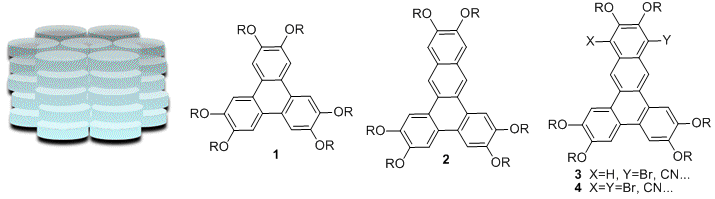
Columnar liquid crystalline phases, where disk-shaped molecules self-assemble into extended π-stacked arrays (Figure 1), are attractive targets for the design of new organic semiconductors. Specifically, the π-π-overlap can facilitate charge transport, while the fluidity of the liquid crystalline phase can be used to prepare aligned films with few defects (which are detrimental to charge transport). The design of new discotic molecules that exhibit columnar liquid crystal phases typically includes a polycyclic aromatic hydrocarbon core with peripheral flexible alkyl chains, such as the hexaalkoxytriphenylenes (1). The corresponding hexaalkoxydibenz[a,c]anthracenes (2) have not been reported, despite the fact that extending the core may promote π-stacking and also lower the HOMO-LUMO gap. One of the primary goals of this research is to prepare a series of dibenzanthracenes and to evaluate their liquid crystalline properties. We previously developed a versatile synthetic approach to haxaalkoxydibenz[a,c]anthracenes (2). Surpisingly, none of the compounds of series 2 exhibited liquid crystalline phases. However, we found that substituents at the 10- or 10-and 13- positions (series 3 and 4, respectively) exhibited columnar mesophases over broad temperature ranges. The nature of the substituent had a profound impact on the liquid crystalline temperature range. While there appear to be several factors influencing the liquid crystal temperature range in compound series 3 and 4, electron withdrawing substituents promote stable mesophases over broad temperature ranges.
Figure 1. Schematic Representation of a hexagonal columnar mesophase, and structures of hexaalkoxytriphenylenes and hexaalkoxydibenz[a,c]anthracenes.
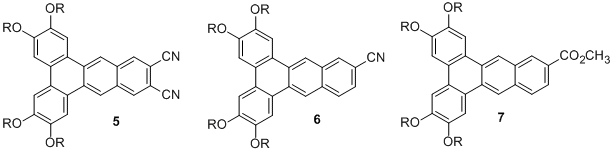
More recently, we have developed the synthesis of dibenzanthracenes bearing different substitution patterns incorporating electron-withdrawing substituents to promote liquid crystallinity. Specifically, we have prepared compounds 5-7 using the synthetic approach we have developed. Preliminary results indicate that series 5 exhibits a relatively broad liquid crystal phase range, series 6 exhibits a very narrow mesophase range, and series 7 does not exhibit any liquid crystallinity. These results are consistent with our previous findings, suggesting that electron-withdrawing substituents promote pi-stacking that is required for the formation of these columnar mesophases.
Figure 2. Recently prepared dibenzanthracenes with electron-withdrawing substituents.
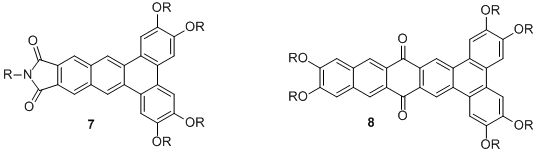
Based on the results we have obtained, our current efforts focus on the synthesis of related PAHs with an electron-deficient aromatic core. For example, we have recently prepared compound 7 bearing an electron-withdrawing imide and an alkyl chain attached at the nitrogen to promote the formation of a columnar phase over a broad temperature range. Our preliminary results indicate that this compound does indeed form a hexagonal columnar phase over a broad temperature range, although a more detailed investigation of the properties of these compounds is underway. We are also in the process of preparing 8, which features an electron-deficient quinone core. We anticipate the quinone core will also promote a stable mesophase over a broad temperature range.
Figure 3. Current target compounds
Over the past year, this grant has directly supported two undergraduate students, who have developed their skills in synthetic chemistry. One additional undergraduate student has contributed to the project, along with a master's student. While one of the undergraduate students and the master's student are continuing their studies, two of the undergraduate students have completed their BSc degrees. One of the undergraduate students directly supported by the project has joined my lab as a master's student, while the undergraduate student indirectly supported by the grant is pursuing her master's at McMaster University.
In summary, we have extended our synthetic approach to dibenzanthracenes to a series of compounds with alternate substitution patterns. We have confirmed that electron-withdrawing substituents promote the formation of stable mesophases and are using these results to design new dibenzanthracene derivatives designed to exhibit columnar mesophases over broad temperature ranges. Ultimately, we also hope to compare the solid-state organization of these materials by X-Ray crystallography in order to draw comparisons between the solid state and the liquid crystal state.
Copyright © 2014 American Chemical Society