58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1052423-DNI10: Sol-Gel Assembly of Hollow Metal Particles Into a New Class of Porous Nanostructures and Their Application in Heterogeneous Catalysis
Indika Arachchige, PhD, Virginia Commonwealth University
Overview: The 2012-2013 funding cycle represents the first year of funding for the proposed research. This award is used to support a postdoctoral fellow for 9 months, 0.9 month of summer salary for the PI, and to purchase supplies required for proposed research. The ACS-PRF award has already made a significant impact on my career as an independent researcher as well as the careers of a graduate student and postdoctoral fellow in my group. This grant provided an excellent start for research in my group. I was able to hire a postdoctoral fellow (Dr. Kulugammana Ranmohotti) to successfully initiate the proposed work. He has made significant progress in the synthesis of metal aerogels and we have presented research finding in 234th ACS National Meeting and 2012 Southeastern Regional Meeting of the American Chemical Society. In addition, the significant research progress achieved by Dr. Ranmohotti and an unsupported graduate student (Mr. Xiaonan Gao) resulted in the first peer-reviewed journal publication from my group.
Scientific Progress: We have been investigating two approaches for the assembly of metal nanoshells into metallic aerogels, namely salt-mediated self-assembly and oxidation-induced self-assembly. To this end, Dr. Ranmohotti investigated that the self-assembly of Au/Ag, Pt/Ag and Pd/Ag hollow particles can be triggered by adjusting the concentration of the ionic byproducts ([Na+] = 0.0024-0.0073 mol L-1) in concentrated colloidal solutions of nanoshells ([M] = 0.005-0.01 mol L-1 where M = Au/Ag, Pt/Ag and Pd/Ag). Consequently, metal hydrogel were obtained after 1, 4, 15, and 28 days when the Na+ ion concentrations are 0.0073, 0.0052, 0.0041, and 0.0028 mol L-1, respectively. In contrast, the formation of metallic gel structures using the oxidation-induced self-assembly has been reported to require 1-4 weeks.
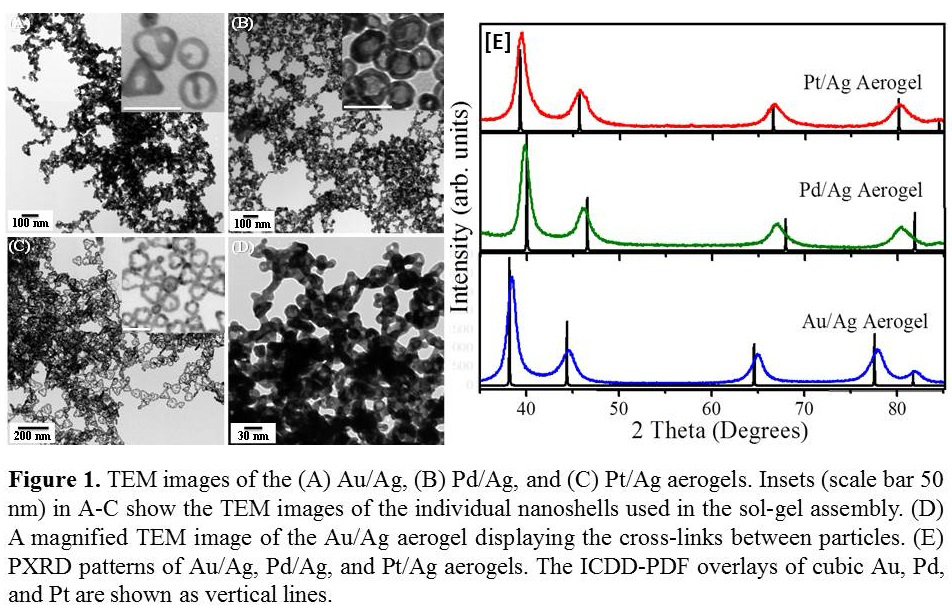
It was found that the salt-mediated self-assembly has no apparent impact on the structure and crystallinity of the nanoshells. The X-ray diffraction (XRD) data of the aerogels are characteristic of their cubic phases with slight shift towards larger (Au/Ag and Pt/Ag) or smaller (Pd/Ag) 2θ angles owing to the formation of respective alloys (Figure 1). The gel morphology is composed of an interconnected network of triangular or nearly spherical hollow particles that have typical dimensions in the same scale as the precursor nanoshells. Additionally, 3-dimensional connectivity between nanosized constituents can be clearly observed specifically, in the dark contrast areas (multiple layers of nanoshells) of the aerogels (Figure 1A-D). The surface areas determined using the BET model were found to be 42, 40, and 32 m2/g for Au/Ag, Pd/Ag, and Pt/Ag aerogels, respectively. The BJH porosimetric plots revealed average pore diameters in the range of 11.8-14.3 nm for all samples. In addition, for Au/Ag aerogels two distinct pore size distributions were observed; a narrow but intense pore distribution centered around 3.8 nm and a much broader size distribution ranging from 15-100 nm. It was found that the narrow pore size distribution observed at 3.8 nm corresponds to smaller hollows observed within the Au/Ag nanoshells, while the latter broad distribution corresponds to meso-to-macro pore network created by the 3-D assembly of nanoshells. The fact that the interior surface of the nanoshells is detectable and accessible can provide an opportunity to tailor the surface area and porosities of the metal aerogels as a function of nanoshell size and to develop customized gel frameworks for specific applications.
Given the demonstrated success of the synthesis of semiconductor gels via oxidative removal of the thiolates from respective nanoparticle colloids, we have also investigated the use of thiol-coated metal nanoshells for gel formation studies. Preliminarily, we have demonstrated in a proof-of-principal experiment that Ag gel structures can be prepared by the oxidative removal of thiolates from glutathione-coated Ag nanoshells using tetranitromethane (oxidant). It was revealed that the ability to transform Ag nanoshells into polymeric gel relies in part on the kinetics by which active sites for the self-assembly become available on the nanoshell surface. If no sites are available because of strict passivation by thiolates, colloids are stable in solution, whereas if too many sites become available at any one time, precipitation can occur. Accordingly, we found that at some minimum ratio of oxidant/thiolate, X = 0.3, no gel is formed. Above X, the rapidity at which gel formation occurs increases with increasing X, until the kinetics are so fast that a precipitate forms in lieu of a gel at ∼12X. Therefore, density, surface area, porosity, and consequently the degree of interparticle interactions can be tuned as a function of the oxidant, amount of oxidant (from X-12X), and the aging time. As-prepared Ag aerogels were black in color and showed a 5% volume loss when compared to hydrogels. Notably, the monoliths of Ag exhibit densities as low as 0.06 g/cm3, representing 0.57% of the density of Ag. Elemental analysis suggest that thiol (sulfur) content is reduced from 35% to ~5% (NSs vs. aerogels), which is consistent with the oxidative removal of glutathione.
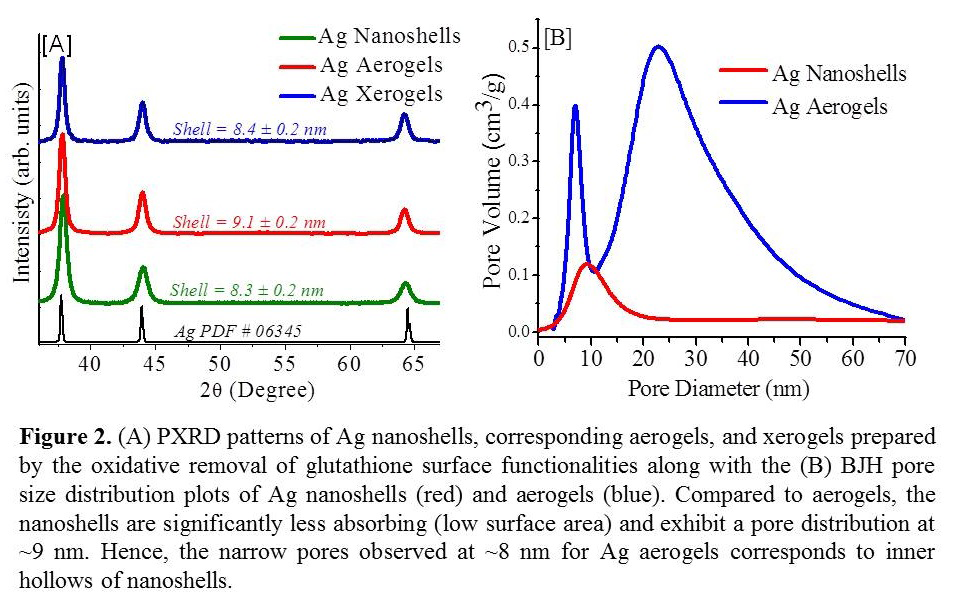
We found that the oxidation-induced gel formation has no apparent impact on the structure and crystallinity of the particles that make up the gel framework. Powder XRD patterns of the Ag aerogels are characteristic of the cubic Ag phase with a slight increase in shell thickness owing to mild thermal sintering during supercritical drying (Figure 2A). The BET surface area is found to be in the range of 118-159 m2/g for Ag aerogels. In contrast, the precursor nanoshells are significantly less porous and exhibit a surface area of ~11 m2/g. The pore diameters calculated based on BJH model revealed values in the range of 13-24 nm for Ag aerogels. Interestingly, two distinct pore distributions were found; a narrow distribution centered at ~8 nm and a much broader distribution ranging from 10-50 nm, which corresponds to hollow diameter of the precursor nanoshells and the meso-to-macro pore network created by the 3-D assembly of nanoshells, respectively (Figure 2B). Over the next year, we will extend this innovative strategy for the self-assembly of other catalytically active nanoscale metals (Pt, Pd, and Ni) into uniquely porous nanoarchitectures and investigate their application in heterogeneous catalysis.
Copyright © 2014 American Chemical Society