58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1052461-DNI10: The Relationship Between the Supercooled Liquid Region, Elemental Enthalpy of Hydride Formation, and Hydrogen Embrittlement in Amorphous Metallic Thin Films
Mary Laura Lind, PhD, Arizona State University
Introduction
Hydrogen is purified from a variety of feedstocks and is used in fuel cells, in the production of commodity chemicals, and for energy generation through the integrated gasification combined cycle. Industrially, hydrogen is primarily produced through the water-gas-shift reaction at temperatures near 820°C. Within the spectrum of membranes available for hydrogen separations (polymeric, inorganic, and metallic), amorphous metallic thin films show promise as lower cost, stable alternatives to expensive palladium membranes. Metallic membranes, which operate by a hydrogen dissociation-solution-diffusion mechanism at temperatures around 300-400°C, are capable of producing extremely high purity hydrogen. However, hydrogen embrittlement of the metallic materials causes a rapid degradation in hydrogen selectivity performance. This is a critical problem facing all metallic membranes, both crystalline and amorphous.
Amorphous metals (also called metallic glasses) have no long-range crystalline atomic order, are in a thermodynamically metastable state, and possess a supercooled liquid region (SCLR). A key feature of amorphous metals is that they have a glass transition temperature (Tg). However, prolonged exposures to temperatures near Tg and in the SCLR can promote crystallization of the amorphous metals which can result in mechanical failure as well as reduced hydrogen permeability. Amorphous alloy systems in the Ni-Zr and Ni-Nb-Zr families exhibit similar hydrogen permeability to that of benchmark Pd-Ag alloy membranes. However, these metallic glasses ultimately fail as a result of hydrogen embrittlement.
In our research, we aim to thoroughly investigate the relationship between Tg, the SCLR, and hydride formation and hydrogen transport properties of amorphous metallic thin films. Specifically, this involves detailed characterization and performance testing of a variety of metallic glasses to systematically evaluate hydrogen separation performance and embrittlement and try to link these performance parameters to fundamental properties of the materials.
Materials preparation
We have selected amorphous metal compositions to investigate that include elements that are known to promote hydrogen transport and have thermal properties to withstand the desired hydrogen separation temperatures. We use Pd-Ag and Ni-Zr as reference materials to compare our results from the different amorphous systems.
 |
We prepared master alloy ingots of the selected amorphous compositions through arc-melting of pure metals purchased from Alfa-Aesar. From the master alloy ingots we synthesized amorphous metallic membranes by splat quenching. Splat quenching is a method to rapidly cool molten metal between two copper plates, it has a cooling rate of 10^6 K/s, creating films approximately 20-40 micrometers in thickness. We have made two hundred splat quenched samples of a variety of compositions.
Materials Characterizations & Testing
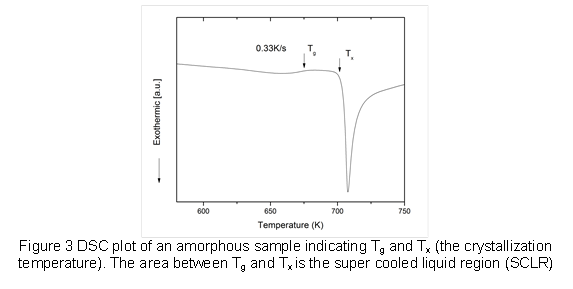
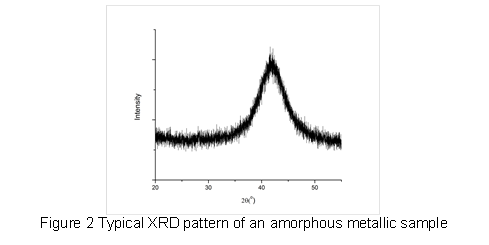
After synthesizing the rapidly cooled metallic films, we used X-ray diffraction (XRD) and differential scanning calorimetry (DSC) to verify the amorphous nature and thermal properties of the as-cast thin film. Figure 2 shows a typical XRD pattern for an amorphous sample with the characteristic broad peak of the amorphous phase. Figure 3 shows a characteristic DSC pattern of an amorphous sample, indicating the glass transition temperature (Tg), crystallization temperature (Tx), and SCLR.
 |
 |
We built a custom hydrogen permeation system that seals our membranes in a stainless steel testing cell with graphite gaskets. A muffle furnace controls the temperature of the system, mass flow controllers regulate the rate of feed gas and sweep gas through the system, and pressure regulators maintain the pressure within the system. We use a gas chromatography (Agilent 7890) to monitor the composition of the permeate gas. Hydrogen permeability is calculated by Sievert's law. We have completed a short study calibrating our system with the Pd-Ag membranes. Recently, we have begun evaluating the hydrogen permeation and embrittlement properties of our amorphous metallic thin films.
Future Work
Our future work focuses on annealing amorphous metallic membranes to different enthalpic states to establish the relationship between the energetic state of the alloy and hydrogen permeability. This will encompass extensive permeability testing and subsequent characterization of the materials through XRD, DSC, X-ray photoelectron spectroscopy, and mechanical testing to evaluate the impact of the hydrogen on the amorphous structure.
Impact
This project integrates the PI's doctoral research on amorphous metals and postdoctoral work in membranes. While the PI has extensive experience in membranes for water purification, this research expands PI's expertise in a new direction: gas separations. This award was the first, externally funded project the PI received as an assistant professor and is critical to her professional development. This award also supports her first graduate PhD student, Tianmiao Lai. Since receiving the PRF DNI grant, the PI has also won a NASA Space Technologies Research Opportunities – Early Career Faculty award and a NSF CAREER award.
PhD student Tianmiao Lai recently passed her comprehensive exam and is now a candidate for the PhD in Materials Science at ASU. At the 2013 North America Membrane Society (NAMS) annual meeting, Tianmiao presented a poster with her preliminary results. Currently, we are preparing publications of our results for submission.
Copyright © 2014 American Chemical Society











