58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND552258-ND5: Application of Parahydrogen Enhanced NMR to Heterogeneous Hydrogenation on Supported Metal Catalysts
Clifford R. Bowers, University of Florida
Helena Elisabeth Hagelin-Weaver, University of Florida
We are pleased to report that the first year of our ACS PRF-ND5 grant has yielded an abundance of extremely interesting new results.
1. Instrumentation Development
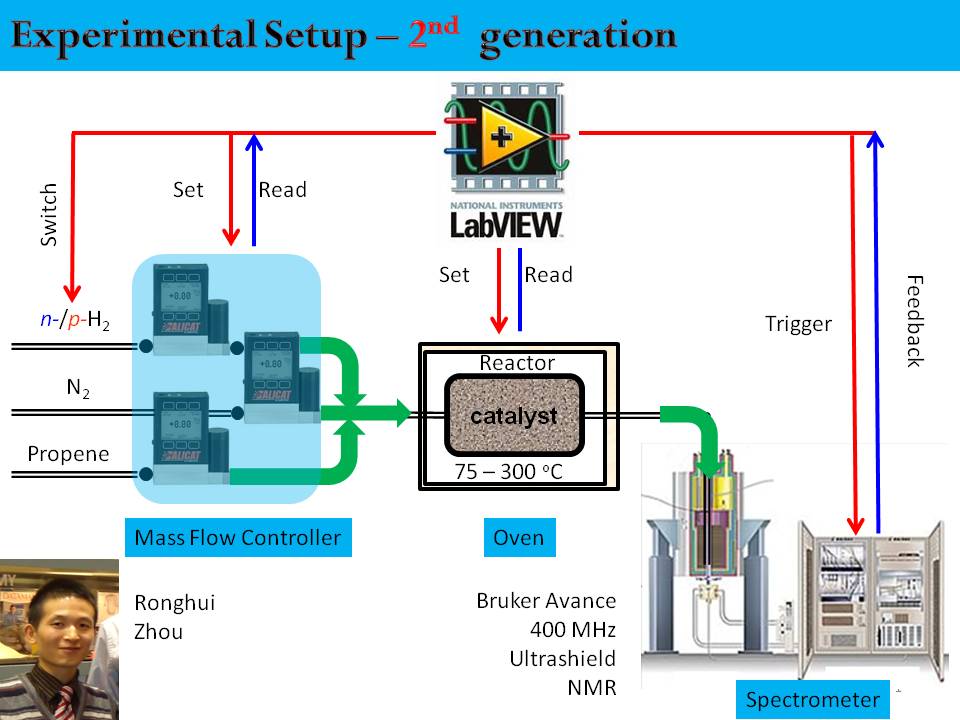
Fabrication of a gas handling system for para-enrichment, storage, and delivery of H2 gas was completed in Fall 2012, and in Spring 2013, assembly and testing of a continuous-flow hydrogenation reactor was completed (see Figure 1).
Figure 1: Schematic of continuous-flow PHIP/ALTADENA hydrogenation reactor.
The system uses three computer-controlled mass flow controls for H2, substrate, and carrier gas (N2) to allow precise and automated control over the reaction conditions. Since these hydrogenation reactions are highly exothermic, accurate temperature monitoring and control of the catalyst bed are critical for obtaining reproducible, valid results. Also, the capability to heat the catalyst bed to a sufficiently high temperature is critically important in the initial catalyst activation step. A National Instruments Labview interface was developed. Our setup has key advantages over the one described in the work by Koptyug et al., the only other group publishing in this area. In particular, we utilize an ex-situ reactor allows better control of temperature and other reaction conditions. Another key advantage of our reactor configuration is that the hydrogenation products are formed at low field and then transported to high field. Massively hyperpolarized proton NMR signals are observed (see Figure 2). These are the PHIP ALTADENA conditions, which yields net alignment with all transitions in-phase within each multiplet. This reduces losses due to destructive interference within the antiphase multiplets when the adducts are generated entirely at high field (i.e. PASDENA conditions).
2. Catalyst Materials
Approximately 20 different oxide-supported Ir and Pt nanoparticle catalysts were prepared.Iridium nanoparticles supported on titanium (IV) oxide (TiO2) were prepared by precipitation of hexachloroiridic acid hexahydrate (H2IrCl6∙6H2O, Alfa Aesar) onto the supports from an aqueous solution by controlling solution pH. The actual metal loading of prepared Ir catalysts was characterized by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). Chemisorption measurements to characterize active surface area, dispersion and particle size of the catalysts were performed at a ChemBET 3000 from Quantachrome Instruments with corroboration from TEM. The data presented below were obtained for the Ir catalyst with properties shown in Table 1.
Metal |
Support |
Loading |
Dispersion |
Particle Size |
|
|
w/w (%) |
(%) |
nm |
Iridium |
TiO2 |
0.544 |
70 |
1.17 |
Table 1.Catalyst information
The reaction shown in Scheme 1 was studied under PHIP-ALTEADENA conditions.
Scheme 1. Hydrogenation of propylene over Ir/TiO2 at elevated temperatures
3. Experimental Details
A U-shaped reactor cell was made from ¼” glass tubing and placed inside a tube furnace mounted next to the bore on top of the NMR magnet. Reaction temperature was monitored with a K-type thermocouple which was inserted into the reactor bed at the outlet of the gas flow and separated from the catalyst with a glass wool plug. 0.05 g of the catalyst was placed on the bottom of the reactor. Catalysts were activated under a stream of H2 (100 ml/min) for 30 minutes at 350 °C, followed by purging under a stream of N2 (300 ml/min) for 15 minutes, and then cooled down under the same N2 stream. p-H2 (50% enriched) was produced in-situ by passing n-H2 through a ¼” copper coil filled with activated charcoal and immersed in a liquid N2. The gas mixture flowed through the reactor at elevated temperatures (50-350 °C), then into the NMR tube which was positioned inside the magnet. To reduce heat generation and time taken to reach steady-state condition, the reactants were diluted in N2. It should be noted that this gas dilution also significantly reduced the possibility of achieving high signal enhancement.
4. Results
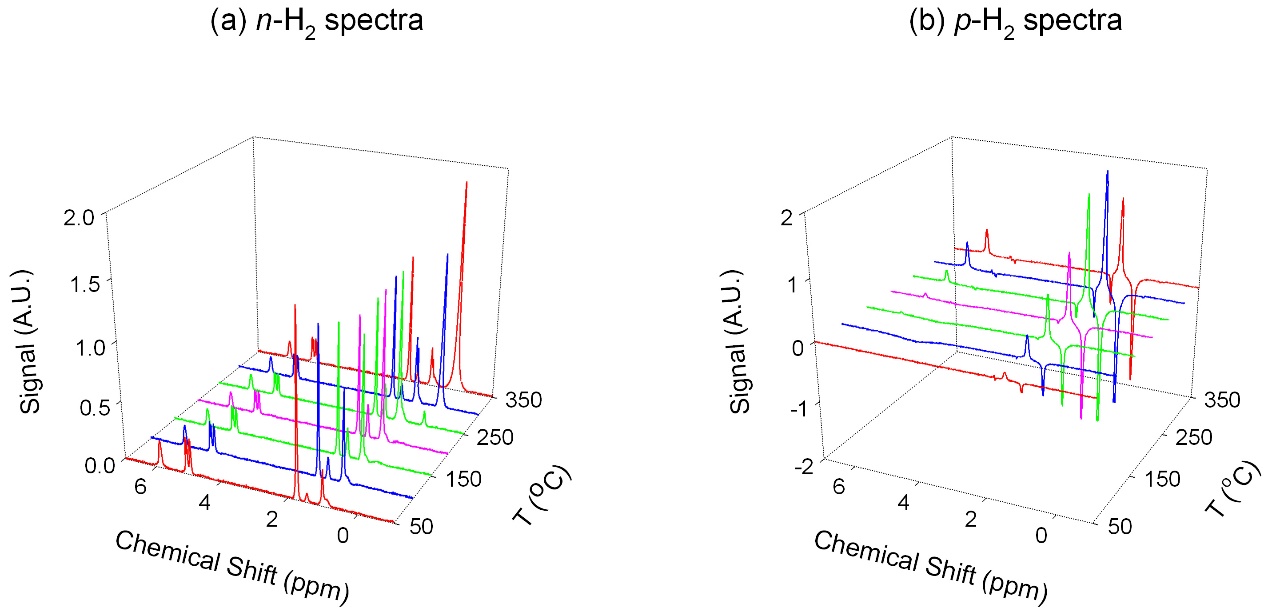
Representative 1H NMR spectra are presented in Fig. 2.
Figure 2. Representative (50-350 oC, 50 oC interval) n-H2 and p-H2 spectra for the temperature dependent studies of propylene hydrogenation (p-H2 spectra obtained by subtracting n-H2 spectra from 50% p-H2). Gas flow rate (total 300 ml/min): N2/p-H2/Propylene=120/150/30 ml/min.
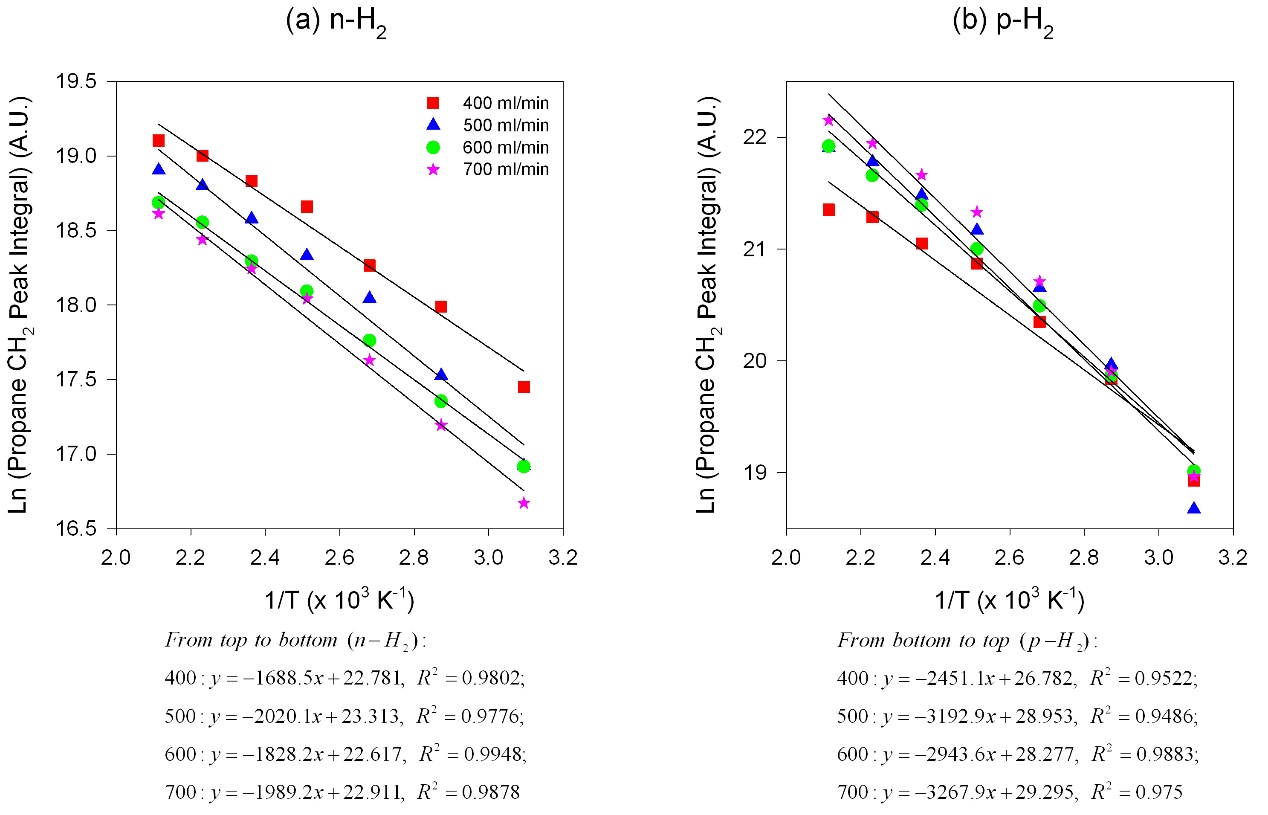
Activation energies for the pair-wise mechanism can be studied by PHIP-NMR, because PHIP-NMR signals stem only from processes that preserve nuclear spin correlation. Activation energy determinations were calculated based on the standard Arrhenius Equation. The results show in Fig. 3 clearly indicated that two different activation energies coexist for n- and p-H2.
Figure 3. The Arrhenius plots of the temperature dependent studies of propylene hydrogenation at a variety of total flow rates. (a) Reactions with n-H2, (b) reactions with p-H2. Total gas flow rates (ml/min): red square-400, blue triangle-500, green dot-600, pink start-700.
The high activation energy for the reaction with p-H2 (17.1 kJ/mol) than that with n-H2 (11.5 kJ/mol) may suggests that the processes pairwise and random additions are distinct. We hypothesize a transitions state with higher energy on the 100 plane than that on the 111 plane, attributed to differing coordination numbers and interactions between reactants and surfaces.
The temperature dependence of propylene hydrogenation is summarized below.
Total flow rate |
Activation Energy |
|
(ml/min) |
(kJ/mol) |
|
Random |
Pairwise |
|
300 |
11.5 |
17.1 |
400 |
14.0 |
20.4 |
500 |
16.8 |
26.5 |
600 |
15.2 |
24.5 |
700 |
16.5 |
27.2 |
Average |
14.8 |
23.14 |
Total flow rate dependent activation energies
The reaction orders provides insights into the reaction mechanisms.
Temperature |
Reaction Orders |
|||||
(℃) |
H2 (propylene: 0.1) |
H2 (propylene: 0.5) |
Propylene |
|||
|
Pairwise |
Random |
Pairwise |
Random |
Pairwise |
Random |
150 |
0.88 |
0.79 |
0.97 |
0.97 |
NA |
NA |
250 |
1.08 |
1.07 |
1.18 |
1.66 |
0.82 |
0.48 |
350 |
1.72 |
1.5 |
2.04 |
2.77 |
0.36 |
0.67 |
Reaction orders under various conditions.
Copyright © 2014 American Chemical Society