58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051019-DNI10: Development of Novel Facilitated Transport Membranes Consisting of Structure-Tunable Molecular Cages for Efficient Olefin/Paraffin Separation
Wei Zhang, PhD, University of Colorado (Boulder)
Summary.
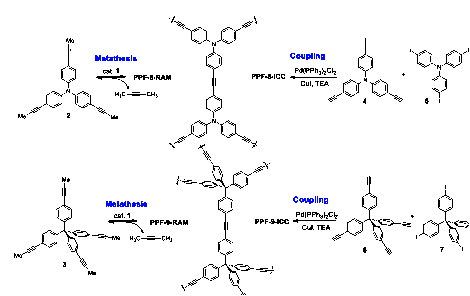
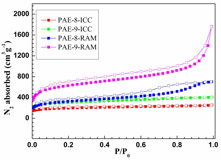
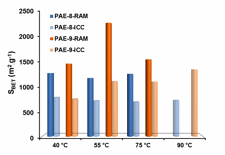
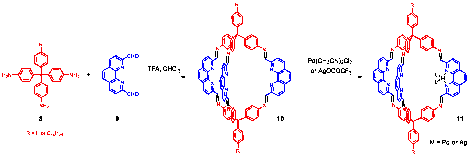
We have successfully synthesized poly(aryleneethynylene) frameworks with high BET surface area through dynamic alkyne metathesis. The frameworks with the same chemical connectivity were also prepared through irreversible cross-coupling reactions. Porous polymer frameworks prepared through alkyne metathesis consistently displayed higher specific surface area and higher thermal stability compared to those frameworks prepared through Sonogashira cross-coupling under similar reaction conditions at various temperatures. Although the frameworks prepared through alkyne metathesis are amorphous at this stage, it is conceivable that such dynamic covalent approach could generate ordered materials in the future.
Copyright © 2014 American Chemical Society