58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI552089-DNI5: Metal Clusters Sequestered in Micropores of Hierarchical Lamellar Zeolites: Protected Active Sites and Enhanced Mass Transport for Heteroatom-Tolerant Catalysts
Dongxia Liu, Univeristy of Maryland
The synthesis and mechanistic characterization of bifunctional catalysts comprised of metal clusters sequestered in micropores of hierarchical lamellar zeolites develops selective and active catalyst formulations for processing compounds in carbon-based fuel feedstocks. The mesopores of hierarchical zeolites enable the processing of bulky molecules while concurrently minimizing the formation of coke by facilitating mass transport. The structural and chemical characterizations and kinetic analysis methods are used to investigate the local geometry and connectivity of the catalytic nanostructures and their catalytic functions to elucidate the effects of protected active sites and bimodal pore topologies of hierarchical zeolites on rates of space demanding catalytic reactions. Our work to date has focused on synthesis of the molybdenum (Mo)-loaded lamellar MFI and MWW hierarchical zeolites and quantification of the active sites for methane aromatization reactions. We have synthesized the zeolite MFI with a variety of crystallite sizes in comparison with the lamellar zeolite structures (Figure 1).
Figure 1. SEM images of (a) MFI-1.4, (b) MFI-0.2, (c) lamellar MFI, (d) MWW, and (e) lamellar MWW, showing the crystallite morphology of each zeolite catalyst.
Table 1. Porosity and structural characterization of MWW and MFI zeolite catalysts before/after Mo loading.
Catalyst | zeolite | Mo loaded zeolite | |||||
crystal size (µm) | surface area (m2/g) | micropore vol (cc/g) | cumulative pore vol (cc/g) | surface area (m2/g) | micropore vol (cc/g) | cumulative pore vol (cc/g) | |
MFI-1.4 | 1.4 | 250 | 0.14 | 0.21 | 199 | 0.11 | 0.18 |
MFI-0.2 | 0.2 | 310 | 0.14 | 0.48 | 257 | 0.11 | 0.44 |
lamellar MFI | 0.0034 | 402 | 0.10 | 0.68 | 355 | 0.09 | 0.61 |
MWW | 0.1 | 375 | 0.18 | 0.55 | 260 | 0.12 | 0.43 |
lamellar MWW | 0.0025 | 481 | 0.23 | 0.56 | 316 | 0.14 | 0.38 |
The textural and acidity properties of the Mo-zeolite catalysts were measured for understanding the distribution and the number of the active sites in the catalysts in the catalytic reactions. The loading of the metal into the catalyst was 4.7% and the sample was treated at 550ºC for 5 hours before the property characterizations. Measured surface area and pore porosity from N2 isotherm data show that lamellar zeolites have higher surface area and pore volume those of the conventional microporous zeolite catalysts. The loading of Mo into the catalyst lowers the pore volume and surface area of the zeolites (Table 1). The measured number of Brønsted acid sites in the zeolites and Mo-loaded zeolites, respectively, by the dimethyl ether titration and elemental analyses show that (i) all of the MFI and MWW zeolites have comparable number of acid sites (ii) the number of free acid sites in Mo-loaded lamellar MFI and MWW were lower than those of Mo-loaded conventional zeolites. Comparing the results in Table 2, we can conclude that the mesoporous structure in lamellar zeolites facilities the dispersion of the metal species that forming a higher number of the interacting Mo-H+ species, which are responsible for the methane activation reactions.
Table 2. Number of active sites (Brønsted acid sites and Mo species) determined by elemental analysis (ICP-OES), DME titration, and XPS analysis methods.
Catalyst | zeolite | Mo loaded zeolite | |||
Si/Al | Si/Al | Si/Mo | Si/Mo | Si/Al | |
MFI-1.4 | 30 | 32 | 25 | 13 | 95 |
MFI-0.2 | 32 | 33 | 27 | 14 | 129 |
lamellar MFI | 30 | 31 | 28 | 32 | 152 |
MWW | 30 | 58 | 26 | 28 | 99 |
lamellar MWW | 32 | 62 | 25 | 34 | 162 |
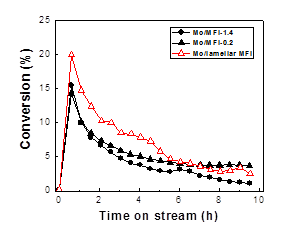
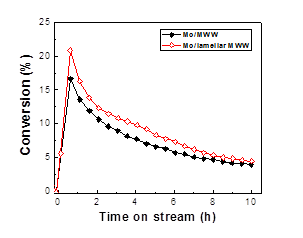
Concurrently, our focus has evolved to study the catalytic performance of the catalyst in the methane aromatization reactions. For all the investigated catalyst systems, the methane conversion sharply increased in the initial period of the reaction (i.e., induction period) and reached a maximum after about 1 h. Afterwards, the methane conversions decreased with TOS. The methane conversion-TOS trend over all the investigated catalysts is similar to that observed in literature. The increase in induction time with increasing zeolite mesoporosity and external surface areas is ascribed to the higher degree dispersion of Mo species in lamellar MFI and lamellar MWW zeolites, which is consistent with that indirectly inferred from the N2 isotherm measurement and DME titrations of Brønsted acidity discussed above. After the reaction induction period, the methane conversion over Mo/MFI and Mo/MWW zeolites followed a sequence of Mo/lamellar MFI > Mo/MFI-0.2 > Mo/MFI-1.4, and Mo/lamellar MWW > Mo/MWW, respectively. Despite their high initial activity, the Mo/lamellar MFI and Mo/lamellar MWW samples deactivated at faster rates compared to the Mo loaded microporous zeolite catalysts.
(a) (b)
Figure 2. Methane conversion with TOS over 4.7 wt% Mo/zeolite catalysts at 983 K, atmospheric pressure, and space velocity of 1600 mL gcat-1 h-1.
In summary, our work has shown that the consequences of meso-/microporous zeolite structures on distribution and activity of active sites (Brønsted acid and metal sites) in molybdenum (Mo) impregnated lamellar MFI and lamellar MWW zeolites enables efficient methane conversion in the initial stage of the reaction. Our current work focused on assessing the fine tuning the mesoporous structures in lamellar zeolite by designed synthesis. This will result in a quantitative assessment of zeolite structure on catalyst function for the synthesis of highly valued-added chemicals from low cost C1 resources. The current research directs the PI to pursue a new direction related to shape selective characteristics of metal/zeolite bifunctional catalytic systems ubiquitous in petrochemical field for methane aromatization, hydrocrakcing, and hydroisomerization of heavy hydrocarbons where the mass transport and catalyst deactivation limit the catalytic reactions. More generally, the PI aims to develop diverse strategies for isolating metal and metal oxide clusters in restrictive environments of hierarchical zeolites to ultimately lead to a new class of catalytic materials that are stable under aggressive reaction environments. Accompanying the development on the PI's career in the catalyst development for efficient catalytic processes, the graduate student working on this project is receiving a systematic training in the field of materials and catalysis. This will lead to the successful education of our next generation of scientists and engineers.
Copyright © 2014 American Chemical Society