58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI352255-DNI3: Armor Plated Ligands: Synthesis and Applications in Catalytic Alkane Functionalization
Vincent Lavallo, PhD, University of California Riverside
Our research program is aimed at the development of ligands that are inert towards undesirable intramolecular reactions with the metal center (cyclometalation) or reactive metal element bonds (insertions). Such intramolecular reactions often result in catalyst decomposition, and have inhibited the development of practical homogenous alkane functionalization catalysts. Our hypothesis is that by appending classical ancillary ligand frameworks with extraordinarily unreactive halogenated icosahedral carborane and or borane clusters, a new generation of “armor plated” ligands (APligands) will be born. In contrast to typical ligands that provide steric protection with hydrocarbon groups, which are susceptible to intramolecular attack by a reactive metal center, AP-Ligands will be immune to such decomposition pathways. This feature coupled with the cluster's highly tunable steric and electronic properties, will render such AP-ligands ideally suited for a range of applications. A large part of our efforts to design AP-ligands requires the development of novel synthetic methods to functionalize the perhalogenated carborane anions. Over the first year of the funding period we have published two manuscripts (Inorganic Chemistry; Full paper, Angeandte Chemie; Communication) detailing the first examples of reactions that allow for the substitution of a B-Cl bond in the extraordinarily inert HCB11Cl11 anion. The ensuing molecules have unusual properties that may be useful for ligand design. An outline of the manuscripts follows.
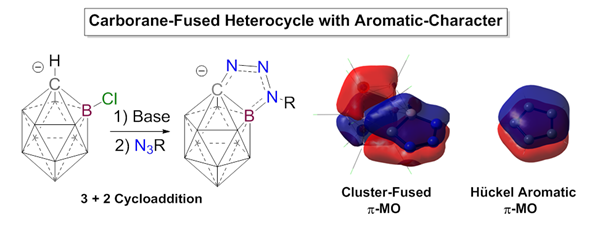 In the first, paper
titled “Click-Like Reactions with the Inert HCB11Cl11-
Anion Leads to Carborane-Fused Heterocycles with Unusual Aromatic Character” we
present the discovery of base induced cycloaddition reactions between the HCB11Cl11-
anion and organic azides that leads to selective C and B functionalization of
the cluster. A single crystal X-ray diffraction study reveals bond lengths in
the heterocyclic portion of the ring that are shortened, which suggests electronic
delocalization. Molecular orbital analysis of the ensuing heterocycles reveals
that two of the bonding orbitals of these systems resemble two of the
doubly-occupied p-MOs of a simple
5-membered Hückel-aromatic, even though they are entangled in the carborane
skeleton. Nucleus independent chemical shift analysis indicates that both the
carborane cluster portion of the molecule and the carborane fused heterocyclic
region display aromatic character. Computational methods indicate that the
reaction likely follows a stepwise addition cyclization pathway. Investigating
the scope of the reaction revealed that similar to phenyl azide, aryl azides,
featuring an electron donating para-methoxy or electron withdrawing para-fluoro
substituent afforded the heterocycles in excellent yields. The reaction also
tolerates sterically demanding mono- and di-ortho substituted aryl azides to
form bulky heterocycles. Interestingly, when n-Butyl azide is used as an electrophile
no heterocycle is formed instead clean C-alkylation occurs with liberation of
LiN3, affording the previously synthesized n-butylated carborane
anion. In contrast when adamantyl azide is used, an electrophile where backside
attack is precluded, the heterocycle forms after extended heating as indicated
by HRMS and 11B NMR spectroscopy.
In the first, paper
titled “Click-Like Reactions with the Inert HCB11Cl11-
Anion Leads to Carborane-Fused Heterocycles with Unusual Aromatic Character” we
present the discovery of base induced cycloaddition reactions between the HCB11Cl11-
anion and organic azides that leads to selective C and B functionalization of
the cluster. A single crystal X-ray diffraction study reveals bond lengths in
the heterocyclic portion of the ring that are shortened, which suggests electronic
delocalization. Molecular orbital analysis of the ensuing heterocycles reveals
that two of the bonding orbitals of these systems resemble two of the
doubly-occupied p-MOs of a simple
5-membered Hückel-aromatic, even though they are entangled in the carborane
skeleton. Nucleus independent chemical shift analysis indicates that both the
carborane cluster portion of the molecule and the carborane fused heterocyclic
region display aromatic character. Computational methods indicate that the
reaction likely follows a stepwise addition cyclization pathway. Investigating
the scope of the reaction revealed that similar to phenyl azide, aryl azides,
featuring an electron donating para-methoxy or electron withdrawing para-fluoro
substituent afforded the heterocycles in excellent yields. The reaction also
tolerates sterically demanding mono- and di-ortho substituted aryl azides to
form bulky heterocycles. Interestingly, when n-Butyl azide is used as an electrophile
no heterocycle is formed instead clean C-alkylation occurs with liberation of
LiN3, affording the previously synthesized n-butylated carborane
anion. In contrast when adamantyl azide is used, an electrophile where backside
attack is precluded, the heterocycle forms after extended heating as indicated
by HRMS and 11B NMR spectroscopy.
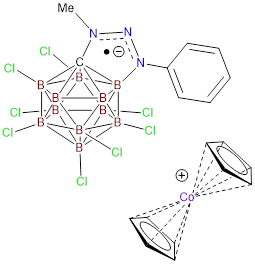
 Redox active
ligands have found an important place in the design of catalysts for the
functionalization of hydrocarbons. In the second paper, titled “Isolation of a
Carborane-Fused Triazole Radical Anion” we present the discovery of a method to
manipulate the electronic properties of carborane anions to render them
amendable to facile chemical reduction. It was postulated that because
carborane fused triazoles from publication #1, such as the N-phenyl substituted
derivative, display some degree of exo-cluster delocalization, these species
might possess interesting redox properties. Cyclic voltammetry (CV) experiments
indicate that compound 1 undergoes no observable oxidations and only
irreversible reductions at large negative potentials. We postulated that the
large potential required to reduce the heterocyclic anion 1 was due to
unfavorable electrostatic repulsion associated with producing a radical
dianion. We reasoned that if the negative charge of 1 were masked, by forming a
zwitterion, its electrochemical properties would be altered in such a way as to
allow facile reduction. Hence, anion 1 was alkylated to afford an
electronically neutral zwitterionic species. In marked contrast to the anionic carborane,
the zwitterion undergoes a clearly reversible reduction at -0.87 V from Fc/Fc+.
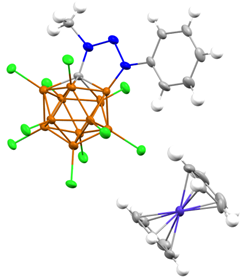
With these encouraging results in hand, we set out to chemically reduce the
zwitterion an isolate the observed radical anion. Thus, a diethyl ether
solution of cobaltocene was added to zwitterionic heterocycle after 12 hours a
green/brown precipitate was isolated. A radical species could be detected by
EPR (one broad signal at g = 2.003, the peak to peak width of the signal is
28.8 G, 28.2 G at 120 K), which is in very good agreement with the value
calculated for the radical anion 3 (g = 2.004). The hyperfine coupling constants
could also be calculated but were not observed experimentally because of the
broad nature of the signal; a fact that may be attributed to the inherent asymmetry
of the five-membered ring. The structure of the radical anion was unambiguously
confirmed by a single crystal x-ray diffraction study, and is the first example
of a stable triazoles radical anion. We are currently incorporating this
structural motif into ligands for hydrocarbon functionalization chemistry.
Redox active
ligands have found an important place in the design of catalysts for the
functionalization of hydrocarbons. In the second paper, titled “Isolation of a
Carborane-Fused Triazole Radical Anion” we present the discovery of a method to
manipulate the electronic properties of carborane anions to render them
amendable to facile chemical reduction. It was postulated that because
carborane fused triazoles from publication #1, such as the N-phenyl substituted
derivative, display some degree of exo-cluster delocalization, these species
might possess interesting redox properties. Cyclic voltammetry (CV) experiments
indicate that compound 1 undergoes no observable oxidations and only
irreversible reductions at large negative potentials. We postulated that the
large potential required to reduce the heterocyclic anion 1 was due to
unfavorable electrostatic repulsion associated with producing a radical
dianion. We reasoned that if the negative charge of 1 were masked, by forming a
zwitterion, its electrochemical properties would be altered in such a way as to
allow facile reduction. Hence, anion 1 was alkylated to afford an
electronically neutral zwitterionic species. In marked contrast to the anionic carborane,
the zwitterion undergoes a clearly reversible reduction at -0.87 V from Fc/Fc+.
With these encouraging results in hand, we set out to chemically reduce the
zwitterion an isolate the observed radical anion. Thus, a diethyl ether
solution of cobaltocene was added to zwitterionic heterocycle after 12 hours a
green/brown precipitate was isolated. A radical species could be detected by
EPR (one broad signal at g = 2.003, the peak to peak width of the signal is
28.8 G, 28.2 G at 120 K), which is in very good agreement with the value
calculated for the radical anion 3 (g = 2.004). The hyperfine coupling constants
could also be calculated but were not observed experimentally because of the
broad nature of the signal; a fact that may be attributed to the inherent asymmetry
of the five-membered ring. The structure of the radical anion was unambiguously
confirmed by a single crystal x-ray diffraction study, and is the first example
of a stable triazoles radical anion. We are currently incorporating this
structural motif into ligands for hydrocarbon functionalization chemistry.
Copyright © 2014 American Chemical Society











