58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051099-DNI10: Thin Film Absorber Solar Cells Using Earth Abundant Materials
Thomas W. Hamann, PhD, Michigan State University
Thin Film Absorber Solar Cells Using Earth Abundant Materials
Hematite constitutes one of the most promising semiconductor materials for the conversion of sunlight into chemical fuels by water splitting. This promise emanates from its unique combination of desirable properties: a 2.2 eV bandgap that allows the absorption of a significant fraction of sun light, an abundance of iron in the Earth's crust, an extremely low materials cost, and electrochemical stability. The effective use of Hematite as a photoelectrode has generally been prevented, however, due to the short minority carrier mobility and lifetime which results in a very short charge collection length. The goal of our ACS-PRF supported research is to investigate the possibility of utilizing thin films of hematite, prepared via atomic layer deposition (ALD), as a way of overcoming the short charge collection length. This work is driven by systematic investigations of the fundamental structure-function relationships of hematite thin films in water splitting applications.
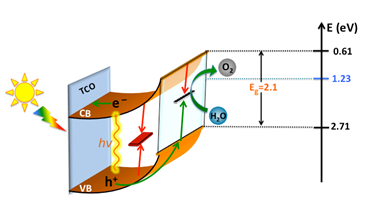
In the first year of our ACS-PRF supported work we showed the importance of understanding the role of surface states and their involvement in water oxidation at the hematite electrode by employing impedance spectroscopy. These results indicated that surface trapped holes may actually be intermediate species in the water oxidation reaction. The surface trapped holes can also participate in recombination with conduction band electrons, however. While the exact role that these surface states play in water oxidation are still unclear, it has become generally agreed upon that surface-state charge recombination at the electrode-electrolyte interface is a competitive reaction with water oxidation that limits the overall efficiency of water oxidation. As a result of our work on this topic, we were invited to write a perspective article where we discussed the most recent results of understanding water oxidation with hematite electrodes and the possible roles played by surface states.
Figure 1. Energy diagram of hematite photoanode in contact with aqueous electrolyte: green arrows represent favorable processes and red arrows represent recombination reactions.
Due to the importance of surface-state mediated reactions, surface coatings on hematite have attracted a lot of recent attention. Since the water oxidation efficiency is determined by the fraction of holes at the surface that participate in the water oxidation reaction rather than recombine, the efficiency should be improved by any combination of increasing the rate of water oxidation and decreasing the rate of recombination. There are many examples in the literature of surface coatings that increase the water oxidation efficiency of hematite, however identification of the mechanism of improvement has not be determined.
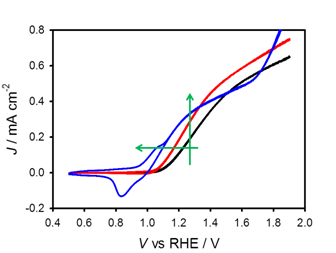
We are continuing our efforts to understand the exact effect of surface coatings by examining a series of materials which we expect to be catalytic or inert to water oxidation. The surface coatings are deposited on hematite surfaces via atomic layer deposition, ALD, which ensures reproducibility and complete surface coverage. For example, we have recently deposited nickel (II) oxide (NiO), a p-type semiconductor and known water oxidation catalyst, onto the surface of hematite electrodes. Using photoelectrochemical measurements we showed that the addition of NiO to the surface of hematite reduced the water oxidation onset potential by over 100 mV and increased the photocurrent at 1.23 V vs RHE. Representative current density, J, vs. applied voltage, V, curves are displayed in Figure 2. There is a very prominent dependence of the J-V behavior, thus efficiency, on the thickness of the NiO deposited. We employed electrochemical impedance spectroscopy (EIS) to understand the cause of the improvement as well as thickness dependence of the NiO coatings. Initial fit results of the EIS data indicate that water oxidation is occurring via Ni(OH)2 that is formed at the electrode surface and it is thus acting as a catalyst, not passivating surface states. Further, the NiO thickness dependence of the J-V curves is largely a result of the ability of charged Ni(OH)2 to control the hematite/catalyst junction (i.e. Fermi-level pinning). These results are currently being written up for publication.
Figure 2. Plots of J-V curves of bare Fe2O3 (black), 1 ALD cycle of NiO coated Fe2O3 (red), and 100 ALD cycle NiO coated Fe2O3 under 1 sun illumination. Arrow represents a decrease in the water oxidation onset (horizontal) and an increase in the photocurrent (vertical) with the addition of NiO.
This ACS-PRF grant has been effectively used to attract and support the work of two graduate students. One of the graduate students defended his PhD in the spring 2013, thanks in part to support from this grant, and is now an EERE postdoctoral fellow at Northwestern University. In addition, this grant has allowed for the support of an undergraduate researcher. This support has allowed her to gain vital research experience which I expect to help her take the next step in graduate school and launch her career. The results from this project have been presented several national and international conferences and resulted in multiple publications in top journals. The results presented in this progress report also formed the basis for funded NSF CAREER Award proposal, which will help support additional students and allow further directions related to this work to be pursued in the future. I was granted early tenure this year which was partially a result of the research this award allowed us to pursue early in my academic career. Thus, the award of this grant, which was my first award, has been instrumental in advancing my career.
Copyright © 2014 American Chemical Society