58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR351716-UR3: Ligands for the Cobalt-Catalyzed Dimerization of Alpha Olefins
Richard D. Broene, Bowdoin College
Linear α-olefins comprise a large, industrially relevant class of organic compounds used as precursors in the manufacture of surfactants, synthetic oils, and co-monomers in a variety of plastic compounds. They are typically produced by one of seven different industrial processes, all of which are full-range processes because they generate a broad distribution of lengths upon oligomerization of ethene. The distribution of linear α-olefins generated typically stretches from C4 to C50. The greatest demand for linear α-olefins, however, is the C6-C18 (1-hexene to 1-icosene) range—where they are used as plasticizers and surfactants. An attractive alternative to recycling the short chain alkenes to the oligomerization reaction is to convert them into higher value, linear α-olefins through a catalyzed dimerization reaction and we reported the first example of linear dimerization in 2005 using Cp*CoP(OMe)3Et BArF as catalyst.
We spent the 2012-13 grant period synthesizing cobalt species that we predict will provide better selectivity for the dimerization process via decreasing the steric bulk of the supporting phosphite ligand. To date we have successfully synthesized several different classes of ligands and those results are reported below.
Planar tethered Ligands
We have successfully synthesized
the Cp*Quinoline Co complex
described described by Enders.[1] Lithium halogen exchange of
2-bromoquinoline (1 via Skraup reaction of 2-bromoaniline with glycerin) with nBuLi and addition to tetramethylcyclopentenone
(2) gave alcohol 3 in up to 40% yield. We found that the
best yields were achieved when the dehydration reaction was done by stirring
the alcohol in a pH 9 NH4OH solution for 30
minutes. Coordination of the ligand to the metal
was accomplished with Co2(CO)8
using 1,3-cyclohexadiene as a hydrogen scavenger. Iodine oxidation to Co(III) displaced the two carbon monoxide ligands and gave
the di-iodo species 5 in 43% yield.
Reduction of this complex to the ethene coordinated
Co(I) species 6
has proven difficult with all attempts so far giving paramagnetic Co(II)
species. We have attempted
reduction of 5 with Zn(Hg), Na(Hg) KC8, BuLi,
and Et2Zn with no success.
We have also attempted to remove the CO ligands of 4 with Me3NO in the presence of ethene
with no success. We are currently
investigating the redox properties of 5 via CV to discern its ability to be
reduced to Co(I).
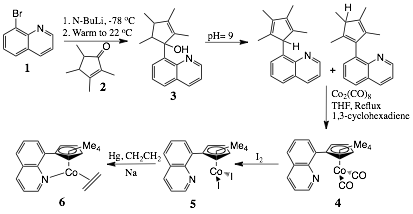
Scheme 1: Synthetic pathway leading to the desired olefin complex 6.
Other planar ligands:
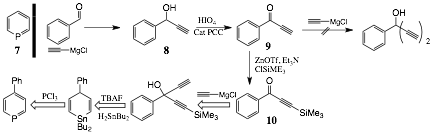
We previously synthesized the phosphabenzene ligand 7 via Ashe's method[2] and found that it had interesting coordination properties but was inconvenient to synthesize and handle. During this last year we began the synthesis of para substituted phosphabenzene derivatives, with a special emphasis on the 4-phenyl derivative. The experimental path, shown in Scheme 2 was the most successful of several attempted. Addition of ethynyl magnesium chloride to benzaldehyde gave the alcohol 8, which was oxidized using periodic acid in the presence of a catalytic amount of PCC to ethynylphenyl ketone 9. Addition of ethynyl Grignard to the ketone was found to deprotonate the terminal alkyne, so 9 was treated with ZnOTf, Et3N and TMSiCl to silylate the alkyne to give 10. We are currently exploring conditions for the addition of the second alkyne to the ketone.
Scheme 2. Synthesis of 4-substituted phosphabenzene.
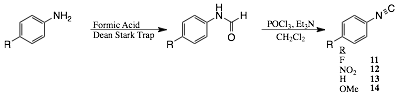
Isocyanides
Finally, we have made considerable progress toward the coordination of isocyanides (RNC) to Cp*Co(ethene)2. Synthesis of a variety of aromatic RNC were undertaken from through formamide intermediates as is shown in Scheme 3.[3] The species were chosen as representative of both electron withdrawing and electron donating groups. With these in hand we have begun to explore their coordination properties to the bis ethene Co complex. Our preliminary results using the electron withdrawing species, 4-F-phenyl (11) and 4-NO2-phenyl, demonstrate that the isonitriles do not displace an ethene ligand when added at equal molar ratios to the Co complex but 11 does show evidence of coordination at 20:1 molar ratios. These investigations are continuing.
Scheme 3: Synthesis of isonitriles.
Student Impact.
Six students have been supported by this grant. Two of the students, Margaret Lammert and David Bean, presented their work at the 245th National ACS meeting in New Orleans (Abstract ORGN 558). The other four students who were supported are all currently college juniors and are continuing their projects this academic year.
[1] Enders, M.; Ludwig, G.; Pritzkow, H., Nitrogen-Functionalized Cyclopentadienyl Ligands with a Rigid Framework: Complexation Behavior and Properties of Cobalt(I), -(II), and -(III) Half-Sandwich Complexes. Organometallics 2001, 20 (5), 827-833.
[2] Ashe, A. J., Phosphabenzene and Arsabenzene. Journal of the American Chemical Society 1971, 93 (13), 3293-3295.
[3] Lu, Z.-l.; Mayr, A.; Cheung, K.-K., Synthesis and Formation of Metal Complexes of 4-Alkynyl and 4-Cyano-2,6-Diisopropylphenylisocyanides. Inorganica Chimica Acta 1999, 284
Copyright © 2014 American Chemical Society