58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI651672-DNI6: The Role of the Support in MAO (Methylaluminoxane) Activated Olefin Polymerization
Eva Zurek, PhD, State University of New York at Buffalo
1. Background
Sinn and Kaminsky first realized that adding water to systems such as metallocene/TMA (trimethylaluminum) caused them to become highly active as ethylene polymerization catalysts. The partial hydrolysis of TMA was thought to result in the formation of MAO (methylaluminoxane), which behaves as an activator in the polymerization process. It is believed that MAO abstracts a methyl or alkyl group from the precatalyst, and coordination of the olefin to the metal center is followed by subsequent insertion into the metal–alkyl bond leading to the growth of the polymer chain. The interest in these types of catalysts stems from their high activity, high stereoselectivity, and the narrow molecular weight distribution of the polymer produced. In the past three decades the serendipidous discovery of MAO and its dramatic effect as a co-catalyst or activator in olefin polymerization has revolutionized industrial polyolefin production. Yet, despite tremendous efforts MAO has thwarted experimental characterization, and is sometimes referred to as a “black–box”. The presence of multiple equilibria between different (AlOMe)n oligomers coupled with the interaction between MAO and TMA has hindered it's experimental structural characterization. The system is also challenging to study computationally: standard GGA-type functionals are not able to accurately calculate the relative energies between different species that may be found in a MAO solution, however it is computationally too expensive to study all but the most simple species involved using correlated wavefunction based methods. MAO harbors many mysteries, but perhaps the most puzzling are the following three:
· what is/are the structure(s) of MAO?
· why is a large excess of MAO necessary in order for polymerization to occur (Al:catalyst ratios of 10,000:1)?
· why does this ratio dramatically decrease when MAO is supported on MgCl2, silica or alumina surfaces (100:1-500:1)?
With support from this grant we are carrying out first-principles calculations in order to answer these questions, and also studying other important catalytic processes.
2.1 Spherical versus Nanotubular MAO: Equilibria and Electric Field Gradients
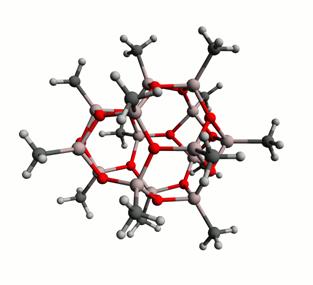
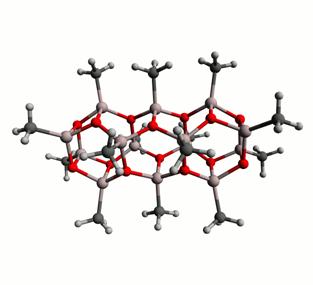
Both computations and experiment suggest that the predominant species in MAO are cage-like polyhedral structures containing three coordinate O atoms, four coordinate Al atoms as well as square and hexagonal faces. These hypothetical (AlOMe)n species are reactive and exhibit latent Lewis acidity (LLA) because of the ring strain present in the cages. As a result, the residual TMA which is present in solution reacts with the cages such that the average C:Al ratio is greater than one. A number of structural alternatives have been proposed, including ‘spherical' and ‘nanotubular' (AlOMe)n polyhedra. For example, two possible (AlOMe)14 oligomers are illustrated in Figure 1. Because the spherical oligomers do not contain many LLA sites, in stark contrast to the nanotubular alternatives, the former were calculated as being more stable than the latter. For this same reason free TMA was found to react preferentially with the nanotubular species. In reality it is likely that there exists a dynamic equilibrium between spherical MAO cages and free TMA, as well as finite MAO nanotubes which have been capped at the ends with TMA. We have performed DFT calculations in order to determine the free energy for this equilibrium and estimated the percent distribution of the various species present in MAO as a function of temperature. Furthermore, we have calculated the NMR chemical shifts and electric field gradients (EFG) for aluminum and oxygen atoms in different species which may be present in MAO, and related them to their geometric environments.
Figure 1: A (left) ‘spherical', and (right) ‘nanotubular' (AlOMe)14 oligomer. The energy of the spherical species has been calculated as being 17.2 kcal/mol lower than that of the tube.
2.2 MAO adsorbed on the Magnesium Chloride Surface
Molecular calculations have been carried out to study the interaction of various species which could potentially be present in a MAO solution with a cluster model for the (100) MgCl2 surface using dispersion-corrected DFT calculations. Favorable interactions include the binding of an O atom in a MAO cage with a five-coordinate Mg atom, as well as bridging methyl-Mg bonds. One possible way in which [(AlOMe)6 · (AlMe)3]+[Cp2ZrMe2]-, a proposed model for the ‘active' species in polymerization, could adsorb to the surface is illustrated in Figure 2. Computations using different surface cuts, and larger surface models are currently underway.
Figure 2: One possible way in which [(AlOMe)6 · (AlMe)3]+[Cp2ZrMe2]- may adsorb to the (100) MgCl2 surface. The binding energy has been calculated as being 39.7 kcal/mol.
2.3 Presentations
This work has led to a number of invited and contributed presentations by the PI, as well as posters by a graduate student supported on this project. The presentations given to date are provided below.
1. “Role of the Support in MAO (methylaluminoxane) Activated Olefin Polymerization”:
· 96th Canadian Chemistry Conference (CSC) in Quebec, Canada; contributing talk, 05/2013.
· 245th ACS National Meeting, New Orleans; contributing talk, 04/2013.
· North East Regional Meeting (NERM) of the ACS, Rochester, invited talk, 09/2012.
2. “Interactions in Cp2ZrMe2-catalyzed, MAO activated heterogeneous polymerization: A Computational Approach.” NERM of the ACS, Rochester, contributing poster, 09/2012.
3. “Electric Field Gradients of the Quadrupolar Aluminum Nuclei in Oligomers of MAO: A Theoretical Study.” University at Buffalo – Chemistry Graduate Students Symposium, contributing poster, 05/2013.
4. “Computations of the Equilibria Between Various (AlOMe)n Oligomers, and their EFG Tensors.” CSC meeting in Quebec, contributing poster, 05/2013.
Copyright © 2014 American Chemical Society