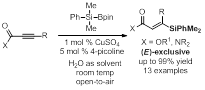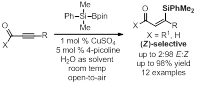58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND350806-ND3: Development of Unsymmetrical Diboron Compounds for Regioselective Diboration and Chemoselective Cross-Coupling Reactions
Webster L. Santos, PhD, Virginia Polytechnic Institute and State University
Organosilicon compounds are versatile building blocks in organic synthesis because of their utility in diverse types of chemical transformations such as C-C bond formation, oxidation to a carbonyl, and alkene formation via protodesilylation. Currently, methods for the preparation of substituted vinylsilanes conjugated to carbonyl groups are scarce and challenging. As a result of our previous studies on the borylation and silylation of α,β-unsaturated carbonyl compounds, we developed a sustainable and ‘green' chemistry protocol for the synthesis of β-silyl-α,β-unsaturated carbonyl compounds. The developed methods has several advantages: (i) it utilizes a catalytic amounts of copper (II) that is stable to oxidation and represents an economical transition metal source, (ii) the reaction is sustainable and ‘green' because it utilizes water as the solvent, and (iii) the reaction is performed open-to-air at room temperature within a few hours.
Via activation of dimethylphenylsilylpinacolborane in the presence of 4-picoline in water at rt under open atmosphere, a series of substituted alkynyl carbonyl compounds afford β-silyl-α,β-unsaturated carbonyl compounds in excellent chemo- and regioselectivity and good to excellent stereoselectivity. With 1 mol % of copper and 5 mol % of 4-picoline, aldehydes react to generate vinylsilanes in 19:81 to 2:98 E:Z ratio and in 51% to >98% yield (Figure 1). Surprisingly, when esters and amides are used as substrates, the (E)-isomers are exclusively observed in 82% to >99% yield (Figure 2).
Figure 1.
Figure 2.
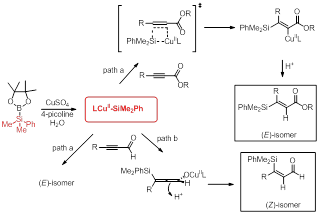
A proposed mechanism for the origin of stereoselectivity is shown in Scheme 1. In situ generated silylcopper species 5 undergoes syn addition across the triple bond of α,β-acetylenic esters to afford vinyl copper species (path a), which is consistent with the complementary Cu-catalyzed conjugate borylation of α,β-acetylenic esters. Protonation of vinyl copper intermediate yields the (E)-isomer. On the other hand, when more electron deficient aldehydes are used, addition of silylcopper species proceeds through two possible pathways: (1) a syn addition similar to that observed with esters in path a generates the E isomer, and (2) a competing conjugate addition in path b that leads to an allenolate intermediate. Because the substituents on the 1 and 3 position of allenolate are orthogonal, protonation occurs on the site opposite the sterically encumbered dimethylphenylsilyl group to provide (Z)-isomer. Since α,β-acetylenic aldehydes selectively provide the Z product, it suggests that conjugate addition is the dominant pathway.
Scheme 1. Proposed Mechanism.
The Ph.D. candidate, Joseph Calderone, who worked on this project, currently has 3 publications. Undoubtedly, support from the ACS PRF is invaluable towards his education and training towards his future goal of working in industry. The publications resulting from this project were used as preliminary data for NSF and IUPAC grant applications.
Copyright © 2014 American Chemical Society