58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR151369-UR1: Utilizing Halogenated Carbonyls to Direct Stereoselective Synthesis
Todd Davis, PhD, Idaho State University
Progress Report 2012-2013
PRF# 51369-UR1
Todd A. Davis
Idaho State University
Part 1: Lewis-base Catalyzed TMSCN & TBDMSCN Additions to Cyclic a-Fluoroketones
Pham, H.; Davis, T. A. Eur. J. Org. Chem. 2013, 3896-3900
Over the past grant cycle, our laboratory has focused on overcoming obstacles in the development of diastereoselective cyanide (trimethylsilyl cyanide, TMSCN) additions to α-fluoroketones. Recently, we disclosed our results for the trimethylsilyl cyanide (TMSCN) additions to cyclic a-fluoroketones in a manuscript published in the European Journal of Organic Chemistry. These reactions work well and proceed in good yields (70- 95%) and diastereoselectivity (up to 22:1) producing the TMS protected
cyanohydrin. Although these reactions work well, they do require long reaction times. To address this problem, our laboratory has focused on using Lewis base catalysis to enhance the reactivity of the TMSCN. In order to utilize Lewis base catalysts our focus
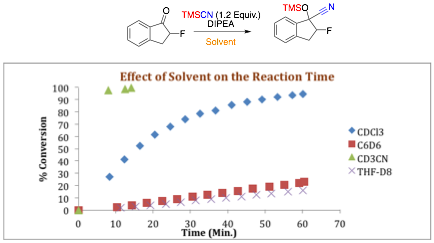
Figure 1: Solvent Effects on Reaction Time for TMSCN Addition Catalyzed by DIPEA
once again was to investigate the role of solvent in the presence of a Lewis base N, N'-diisopropylethylamine (DIPEA) (Figure 1). Using DIPEA as the Lewis base, a variety of solvents were screened and the reaction time decreased in the following order: THF>benzene>chloroform>acetonitrile. This solvent study mirrors those developed by Denmark in the Lewis base catalyzed allyltrichlorosilane addition to aldehydes. Using acetonitrile as the solvent of choice, we next investigated the role of
Figure 2: Role of Lewis base on Reaction Time in TMSCN Addition to a-Fluoroindanone
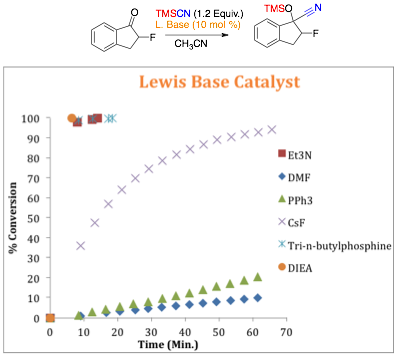 the Lewis base in the TMSCN addition to a-fluoroindanone As seen in Figure 2 ,the reactions
work best in the presence of a nitrogen (Et3N & DIPEA) or
phosphorous (n-Bu3P) containing Lewis base. Based on these results,
diisopropylethylamine (DIPEA, Hunig's base) was chosen as the Lewis base of
choice due to its stability in comparison to the highly oxidizable phosphine
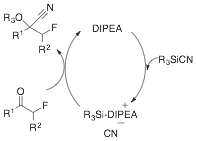
bases. The addition of Lewis base
increases the rate of cyanide dissociation from the organosilanes forming a
contact ion pair (Scheme 1). After
ionization of the cyanide the addition occurs forming TMS-protected cyanohydrin
in good yield and fast reaction times.
the Lewis base in the TMSCN addition to a-fluoroindanone As seen in Figure 2 ,the reactions
work best in the presence of a nitrogen (Et3N & DIPEA) or
phosphorous (n-Bu3P) containing Lewis base. Based on these results,
diisopropylethylamine (DIPEA, Hunig's base) was chosen as the Lewis base of
choice due to its stability in comparison to the highly oxidizable phosphine
bases. The addition of Lewis base
increases the rate of cyanide dissociation from the organosilanes forming a
contact ion pair (Scheme 1). After
ionization of the cyanide the addition occurs forming TMS-protected cyanohydrin
in good yield and fast reaction times.
Scheme 1: Potential Catalytic Cycle for TMSCN Addition to a-Fluoroketones
Adding DIPEA in acetonitrile decreased the reaction time from 24 hours to less than 5 minutes without significantly affecting the yield or stereoselectivity. Optimized reaction conditions therefore favored conducting these reactions in acetonitrile using 10 mol % of DIPEA for the additions.
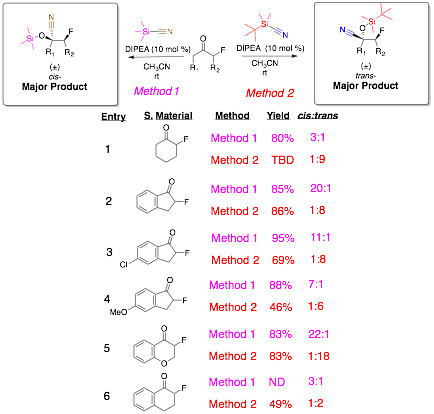
Next, we investigated the role of nucleophile on the stereochemical outcome and yield. Using tbutyldimethylsilyl cyanide (TBDMSCN) under the same reaction conditions reversed the stereochemistry in the product distribution. The product distribution in the TBDMSCN reaction now produced the trans- stereoisomer as the major product whereas the TMSCN nucleophile favored the cis- isomer. We have begun to investigate the substrate scope of these reactions and found that the reaction works well for both five and six membered rings (Table 1). In all cases (Table 1, Entries 1-6) the stereochemistry was directed by the nucleophile chosen. These newly developed methods will provide synthetic chemists methods to predetermine the stereochemical outcome of the reaction based on the nucleophile. The substrate scope will be completed and a submission to the Journal of Organic Chemistry is anticipated for publication of these methods. Currently, we are investigating the mechanism using computational chemistry and low temperature NMR (29Si, 19F, 13C, & 1H). It is our hypothesis that using these methods we will be able to deduce the role of the organosilane nucleophile on the stereochemical outcome and expand nucleophilic additions to a-fluorinated imines.
Table 1: Substrate Scope for TMSCN & TBDMSCN Additions to a-Fluoroketones
Part 2: Lewis-base Catalyzed TMSCN & TBDMSCN Additions to Cyclic a-Chloroketones
The second project that our lab has focused on during this grant cycle is the TMSCN additions to a-chloroketones. We have optimized reaction conditions and found the reaction work well and proceed in modest selectivity and excellent conversion
Scheme 2: TMSCN Additions to a-Chloroketones
(Scheme 2) in a mixture of CH3CN/DMF. Unfortunately, similar to our results in the addition to a-fluoroketones the reaction is slow. Currently, we are investigating the role of solvent and Lewis base on reaction time and selectivity. Based on the results from the addition to a-fluorinated ketones we would expect that these reactions would be strongly dependent on the chosen solvent favoring acetonitrile. With the completion of the solvent studies we will investigate using a variety of nitrogen (DIPEA) and phosphorous (n-Bu3P) containing Lewis base catalysts. Once optimal reaction conditions have been developed the role of nucleophile will be initiated. Our initial results display the same trend as the addition to the a-fluoroketones and display a reversal of the stereochemistry when the organosilanes is changed from TMSCN to TBDMSCN. We will investigate scope and investigate the formation of aziridines and azetidines using these substrates as synthetic precursors.
Student Involvement:
This fiscal year four students funded in our research lab by the ACS-PRF grant. The students were involved in all aspects of the preparation and method development for both parts 1 & 2. Two of these students will continue during the school year and will graduate in the spring of 2014. Both graduating students will pursue graduate studies in chemistry/biochemistry. The remaining student will continue to work next summer and graduate in the spring of 2015.
Copyright © 2014 American Chemical Society