58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: B447422-B4: Synthesis, Thermal, and Photochemical Reactivity of Highly Conjugated Enediynes
John D. Spence, California State University (Sacramento)
Goals: Thermal and photochemical Bergman and related cyclizations of (Z)-hex-3-ene-1,5-diynes, or enediynes, have a wide range of applications from DNA cleaving agents to materials chemistry. Our group is currently exploring the effect of increased conjugation on enediyne reactivity. During the term of this grant, we have developed multiple routes to prepare highly conjugated enediyne chromophores to examine chemical and physical properties of the extended enediyne unit.
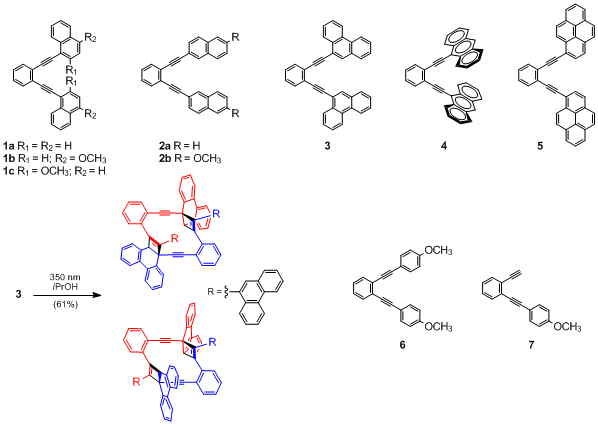
Results: To examine the effect of extended chromophores appended to the enediyne alkyne we prepared a series of highly conjugated arylethynyl arenediynes (Scheme 1). The arylethynyl arenediynes 1-5 all display strong absorption bands ranging from 340 to 430 nm, with two intense emission bands from 400 to 450 nm and fluorescence quantum yields from 0.36 to 0.95. Upon irradiation at 300 nm, only 2b was found to undergo photo-Bergman cyclization in 32% yield. This result, coupled with the lack of reactivity of 2a, indicates an interesting substituent effect from the electron donating methoxy group. In our hands the photo-Bergman cyclization of 2b affords a higher yield of product than 1,2-bis(phenylethynyl)benzene. Irradiation at 350 nm did not lead to any photo-Bergman or related C1-C5 cyclization. Alternatively, 1a, 1b, and 3 were found to undergo a previously unreported [2+2] photodimerization (illustrated for 3). While the yields were relatively low for 1a (11%), improved yields were observed for 1b (47%) and 3 (61%). Interestingly, concentration dependent fluorescence data for 3 suggests excimer formation that may lead to the improved yields compared to 1 which did not show evidence of excimer formation. Unfortunately, pyren-1-yl derivative 5 displays no photochemical reactivity while anthracen-9-yl derivative 4 shows complex reactivity at 300 nm, 350 nm, and 419 nm.
Scheme 1. Arylethynyl Arenediynes
To further examine the influence of electron donating substituents, we expanded our original set of compounds to include methoxyphenyl derivatives 6 and 7 (Scheme 1). Compound 6 undergoes highly reproducible photo-Bergman cyclization at 300 nm in 15-20% yield (again a significant improvement compared to ~5% for 1,2-bis(phenylethynyl)benzene). The mono-substituted derivative 7 affords similar yields of photo-Bergman cyclization product (~15-20%), however, 7 only requires 1.5 hours of irradiation time for complete consumption of starting material compared to 24 hours for 6 and 1,2-bis(phenylethynyl)benzene under identical conditions. With significant improvement in yield and reduced reaction time for 6 and 7, we plan to further examine photo-Bergman cyclization of electron rich phenylethynyl enediynes.
In a second approach to extend conjugation to the enediyne core we prepared a series of aryl-fused quinoxalenediynes 8-14 to examine the effect of extended benzannelation on thermal reactivity (Scheme 2). In solution, 8, 11, and 14 were found to undergo thermal Bergman cyclization as expected. To provide further insight into Bergman cyclization energetics, we conducted a detailed computational study on quinoxalenediynes 8-14 to compare changes in cyclization enthalpy barrier, reaction enthalpy, and barrier for retro-Bergman ring-opening. With extended conjugation, 8-14 were found to have similar activation barriers toward C1-C6 Bergman cyclization; however, with further increase in benzannelation the cyclization was found to become increasingly more endothermic compared to 1,2-diethynylbenzene. This endothermicity subsequently results in a decrease for the retro-Bergman ring-opening enthalpy barrier. Furthermore, the orientation of extended benzannelation was found to have a significant effect on the cyclization endothermicity as isomer 15 exhibited a 6.9 kcal/mol decrease in cyclization enthalpy compared to 8 due to increased aromatic stabilization energy in the respective angularly versus linearly fused azaacene cyclized products. To further examine this system the parent 8 and phenanthrene derivative 11 were converted to the corresponding 6,7-bis(phenylethynyl)quinoxaline derivatives. Upon heating the phenylethynyl derivative of 8 at 200 °C an unoptimized mixture of C1-C6 as well as C1-C5 cyclization products were obtained. In addition, photochemical activation of the phenylethynyl derivative of 11 produced the C1-C5 cyclized product in an unoptimized yield of 5%. Future computational and experimental work on quinoxalenediynes will examine the role of benzannelation (both extent and orientation) as well as alkyne substituent (H versus Ph) on C1-C6 and C1-C5 thermal and photochemical cyclization pathways.
Scheme 2. Aryl-Fused Quinoxalenediynes
Finally, we have also prepared and studied the novel enetriyne 16 and enetetrayne 17 derivatives which contain phenylbutadiynyl units as a means to extend conjugation (Scheme 3). X-ray crystal structures provide c··d distances of 4.039Å and 4.297Å for 16 and 17, respectively. While both 16 and 17 give strong exotherms by DSC, only 16 was found to undergo thermal Bergman cyclization in 11% yield. Enetriyne 16 is the first example of an extended enediyne containing a butadiynyl linkage in which an isolated Bergman cyclization product has been obtained. Unfortunately, 16 and 17 did not afford photocyclization products as each derivative quickly degraded/polymerized upon irradiation at 300 nm and 350 nm. Future work may look toward incorporating electron donating methoxy substituents onto 16 and 17 to determine if enhanced thermal/photochemical yields can be achieved as observed for 2b (Scheme 1).
Scheme 3. Enetriyne and Enetetrayne Derivatives
Impact: Through the term of this grant, 23 undergraduate chemistry majors have been involved in this research program with each student working an average of 4 semesters/summers. Their work has led to 35 off campus student presentations (including 10 at National ACS meetings) while 6 students are listed on published articles with an additional 7 student co-authors on manuscripts currently in preparation. From this group of students, 3 are currently in Ph D programs, 6 are employed in local chemistry related industry, and 5 are currently in medical/pharmacy/dental programs (8 still enrolled). For these students, participation in research provides a valuable learning experience that cannot be matched in the classroom. Participation in summer research would not be possible without support from this grant. Funding from this grant has also helped the PI obtain NSF-MRI funding, California State University Program in Education and Research in Biotechnology (CSUPERB) funding, as well as a number of campus awards, grants, and release time to provide additional faculty/student support.
Copyright © 2014 American Chemical Society