58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND751827-ND7: Photolabile Protecting Group-Based New Method for the Development of Photodegradable Polymers
Pengfei Wang, University of Alabama at Birmingham
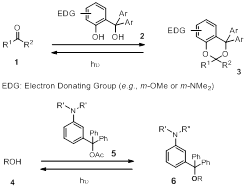
Photolabile protecting groups (PPGs) are protecting groups to be removed with photo irradiation. Their removal is typically under neutral and reagent-free conditions, and can be controlled with high spatial and temporal precision. These features are important to a broad spectrum of applications including polymer science. As a unique external stimulus, light offers a different dimension of controlled polymer property tuning that would be difficult to realize using other stimuli. In addition to the general advantages of using photo stimulus, PPGs will release various well defined chemically active functional groups pendant from the polymer framework, leading to more options for chemical manipulation /functionalization of the polymers. Thus the repertoire of functional materials can be significantly enhanced. However, research in this area is mostly limited to using 2-nitrobenzyl (2-NB)-derived PPGs. Application of novel PPGs with different chemical/photochemical properties in polymer science will provide new opportunities in functional polymeric material development. We have recently developed some robust carbonyl- and hydroxyl-PPGs (Scheme 1). For example, a carbonyl compound (1) can be protected by our new PPG reagent 2 and be converted to an acetal/ketal (3), and an alcohol (4) can be protected as ether by the new PPG reagent 5. Upon UV irradiation, the acetal/ketal 3 and the ether 6 release the carbonyl compound and the alcohol, respectively. These new PPGs are structurally simple, easy to prepare and use, and can be removed in high efficiency under various conditions including in water without excluding air. They are stable under indoor lighting and withstand various chemical treatments. For carbonyl protection, selective removal of different carbonyl PPGs has been achieved by changing irradiation wavelength. Removal of some carbonyl and hydroxyl PPGs can also be successfully carried out with irradiation of sunlight, which is a particularly useful feature for large scale and low cost applications. These features make our PPGs promising for their application in polymer science.
Scheme 1 PPGs featuring benzylic C-O bond cleavage.
We first explored application of the new PPGs in the development of photoresponsive block copolymers (BCPs). BCPs have widespread applications. For example, self-assembly of BCPs has been developed as one of the best strategies for the preparation of porous polymers. In making nanoporous thin films, the underlying strategy consists of the self-assembly of the BCP into the desired structures on a surface followed by removing the sacrificial polymer segment. There are two strategies to remove the sacrificial polymer: either degrading it to small molecular fragments or disconnecting it from the BCP at the junction between the microphase-separated blocks. The latter is deemed more general and flexible because it relys on the chemical nature of the linker rather than the sacrificial polymer. The interest in using PPG-based cleavable joint in BCPs in this regard has grown rapidly.
We designed and synthesized new amphiphilic polystyrene-block-poly(ethylene glycol) (i.e., PS-l-PEG) BCPs bearing our novel PPG-based photocleavable joints. By removing the sacrificial PEG domain of the new PS-l-PEG BCPs under mild irradiation conditions, the carbonyl- functionalized PS domain is obtained. Amphiphilic BCPs, in particular, PS-b-PEG based systems are important in making defect-free porous thin films. It has been recently demonstrated that by removing the sacrificial PEG domain, functional groups, i.e., thiol, carboxyl, amino, and hydroxyl amino group, can be left on the PS domain. The resulting thin film can be further functionalized through these exposed functional groups. We envision that the carbonyl-functionalized porous thin films can be more biochemically/biomedically relevant and useful in that carbonyl group can effectively interact with amino groups of various biological molecules via imine formation.
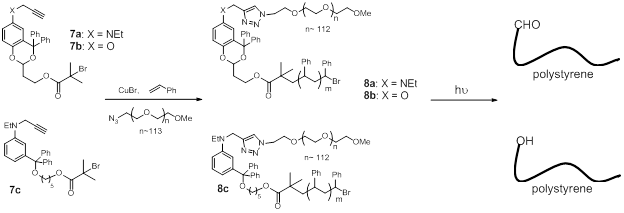
Thus, with the easily synthesized photolinkers 7a-7c derived from our representative carbonyl- and hydoxyl-PPGs, a one-pot copper (I)-catalyzed simultaneous atom transfer radical polymerization (ATRP) and azide-alkyne click reaction (CuAAC) produced the BCPs 8a-8c (PS-li-PEG).
Scheme 2 PS-l-PEG BCPs bearing PPG-based photocleavable linker
Irradiation of the BCP 8a in solution (1 mM in THF/H2O) with a Pyrex-filtered 450 W medium pressure mercury lamp cleaved the BCP within 5 minutes. Production of the product carbonyl-functionalized polystyrene is evidenced by appearance of the CHO signal at 9.5 ppm. NMR, GPC and IR analysis also confirmed photocleavage of the BCP. A Rayonet photoreactor equipped with 350 nm lamps took 3 h to achieve 94% cleavage, and sunlight exposure (a local UV index <5 during the reaction time) took 6 h to reach 91% cleavage. Similar results were also obtained from irradiation of 8a in DMF/H2O. The BCPs 8a of different molecular weights cleaved with about the same efficiency as were achieved in releasing small molecule carbonyl compounds from protection of the parent PPG. Photocleavage was also performed in solid state. A polymer film of 8a (1 mg BCP/200 cm2) irradiated with a Pyrex-filtered 450 W medium pressure mercury lamp for 5 min completed 82% cleavage. Exposre of the polymer film to sunlight (a local UV index 9 during the reaction time) for 1 hour cleaved 51% and 2 hour cleaved 87%. By using the same one-pot copper (I)-catalyzed simultaneous ATRP and CuAAC, BCPs 8b (PS-l2-PEG) and 8c (PS-l3-PEG) were also produced. Irradiation of 8b in solution (1 mM in THF/H2O) with a Pyrex-filtered mercury lamp led to nearly complete cleavage of the BCP. In contrary to 8a, the BCP 8b is stable under 350 nm irradiation. Sunlight cleaved only 25% of the BCP 8b after 6 h exposure. Irradiation of the BCP 8c in solution (1 mM in THF/H2O) with the Pyrex-filtered mercury lamp resulted in 94% cleavage in 30 min. No cleavage was observed with 350 nm irradiation. Under sunlight for 6 h, the BCP cleaved 81%.
In conclusion, we have synthesized new photoresponsive block copolymers containing photocleavable linkers derived from the novel carbonyl- and hydroxyl- PPGs. Under mild irradiation conditions, the joints can be cleaved efficiently to produce carbonyl- or hydroxyl-functionalized PS domain. Selective cleavage of different BCPs by changing the irradiation wavelength has been demonstrated. These photoresponsive BCPs should be potentially useful in a variety of applications.
Copyright © 2014 American Chemical Society