58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR950161-UR9: Experimental Study of Transport Phenomena of Evaporating Fuel Films
Peter L. Kelly-Zion, PhD, Trinity University
Christopher J. Pursell, PhD, Trinity University
The overall goal of this project is to experimentally investigate the thermal and mass transport processes controlling the evaporation of sessile hydrocarbon drops. Through systematic experimentation during which the relative influences of the transport processes are controlled, this project is providing a significantly better understanding of the transport behavior involved in the evaporation process.
During the past year, there were two major achievements in our studies of the roles of vapor phase diffusion and natural convection in the evaporation of a sessile drop. We developed a correlation for the evaporation rates of sessile hydrocarbon drops having a broad range of volatilities, vapor densities, and drop sizes. This correlation was developed for conditions in which the evaporation is influenced by both diffusive and naturally convective transport of the vapor, which are conditions that only are addressed by a couple of published correlations for water. Our correlation is valuable because it provides a simple expression which can replace the need to solve a sophisticated computational model of the evaporation process. More importantly, the correlation provides insight into the nature of the complex coupling between the effects of diffusion and natural convection and thereby helps to promote a better understanding of the vapor phase transport behavior.
The second major achievement of this past year was the final development of an experimental technique to measure the vapor distribution above an evaporating sessile drop. These measurements, which are the first quantitative measurements of the vapor distribution, are important for understanding how diffusion and natural convection in the vapor phase are influencing the evaporation of a sessile drop. Due to the importance of the characteristics of the vapor distribution near the drop surface in determining the nature of the evaporation process, the measurements also are important for validating computational models which are used to study a wide variety of characteristics of the evaporation process. Furthermore, with quantitative knowledge of the vapor distribution, the distribution of the diffusive flux can be computed. In future work, the distribution of the convective flux will be computed from knowledge of the diffusive flux and the overall evaporation rate.
Correlation Development
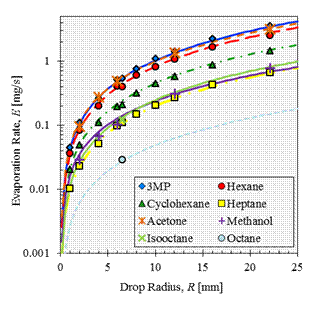
A correlation for sessile drop evaporation under the conditions of both diffusive and naturally convective vapor transport was developed. The correlation is based on experiments conducted with eight hydrocarbons, which provide a factor of 16.6 variation in volatility as indicated by the equilibrium vapor pressures, a factor of 3.6 variation in molecular mass, and a factor of 2.2 variation in mass diffusivity, and thus the correlation is applicable for liquids having a broad range of properties. The correlation predicts the evaporation rates to within a root-mean-square (RMS) error of 6.5% over the broad range of conditions. A comparison between measured evaporation rates and those computed by the correlation is presented in Fig. 1.
Figure 1. Measured and computed evaporation rates of each of the eight components studied as a function of drop size. Measured values are given by the datum symbols and the computed values by the lines. Error bars are smaller than the datum symbols.
The above correlation is applicable to evaporation in air at atmospheric pressure. We have also conducted experiments in an enclosed vessel with a variety of background gases and over a range of pressures in order to extend the range of the influences of diffusive and naturally convective transport mechanisms. We are in the process of studying this enclosed vessel data to improve our understanding of the coupling between diffusion and natural convection, and we will attempt to use this data to derive a general correlation for a sessile drop evaporation.
Vapor Distribution Measurements
An experimental technique that combines infrared spectroscopy and computed tomography was developed to measure the vapor concentration distribution surrounding an evaporating sessile drop. The technique was used to measure the vapor distributions surrounding drops of hexane and 3-methylpentane (3MP), and, to our knowledge, these are the first such measurements to be published.
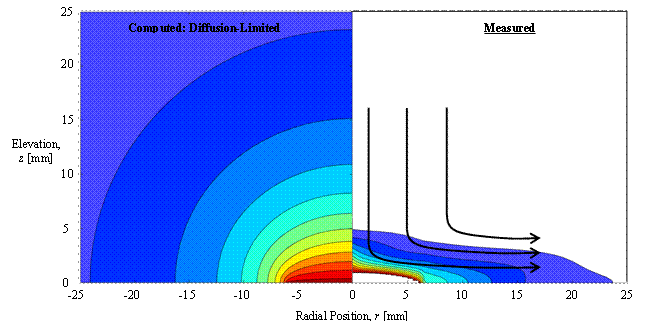
The measured vapor distributions of hexane and 3MP are very similar, and they both differ significantly from the distributions that would result from diffusion-limited evaporation. A comparison of vapor phase distributions above a sessile drop of hexane is shown in Fig. 2. The distribution presented on the left is computed assuming vapor transport occurs solely by diffusion. The distribution presented on the right is the measured vapor distribution and the arrows indicate a hypothesized convective flow.
Figure 2. Comparison of vapor distributions for a hexane drop. The distribution for the condition of diffusion-limited vapor transport is given to the left of the central axis and the measured distribution is given to the right of the axis. Hypothesized streamlines of the buoyancy-induced air and vapor flow are shown by the arrows over the measured vapor distribution.
The differences between the measured and the diffusion-limited distributions are attributed to the effects of natural convection. It is hypothesized that the convective flow pushes the vapor down and radially away from the drop, while also diluting the vapor cloud with air. As a consequence, the concentration gradient at the drop surface is increased. Both the motion of the vapor-air mixture and the concentration gradient at the drop surface can affect the motion of the liquid drop and the distribution of the evaporative flux, two key characteristics that can influence practical applications of sessile drop evaporation, e.g. self-assembly and surface patterning.
In future work, experiments will be conducted for methanol, which has a molecular weight approximately equal to that of air and a much greater coefficient of diffusivity in air than that of either hexane or 3MP. Because of those differences, we expect the measured vapor distribution for methanol to be approximately equal to the distribution that is computed for diffusion-limited evaporation.
Copyright © 2014 American Chemical Society