58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR252201-UR2: Speciation and Sequestration of Rhenium in Sulfidic and Polysulfidic Natural Waters
Trent Vorlicek, PhD, Minnesota State University Mankato
Project Objectives: The purpose of this project is to quantify equilibria and kinetic constants for the formation of thioperrhenates (i.e., ReVIIO3S-, ReVIIO2S2-, ReVIIOS3-, and ReVIIS4-) and reduced Re-polysulfido anions (e.g., ReV(S4)(S4)S-) from perrhenate (ReVIIO4-) in sulfidic and polysulfidic solutions. While Re deposition is believed to involve reduction, the chemical pathway to Re reduction remains undefined. Because the thioperrhenates are more reductively labile than ReO4-, they may be important intermediates in the pathway to Re deposition. S0-donors (i.e., Sn2-, S8(aq), organic polysulfides) can induce Re reduction via Re-polysulfido formation. The project has developed a reverse phase ion pair chromatography (RP-IPC) method for separating and quantifying ReO4-, ReO3S-, and ReS4-; ReO2S2- and ReOS3- are kinetically unstable. Because Re has two stable isotopes (185Re and 187Re), RP-IPC may be used in the future to quantify isotopic fractionation occurring as ReO4- transforms into ReS4-. Coupling the RP-IPC to ICP-MS may also allow for quantifying Re speciation within natural sulfidic waters. Present results also indicate that RP-IPC may be used to isolate Re-polysulfido anions.
Research Progress:
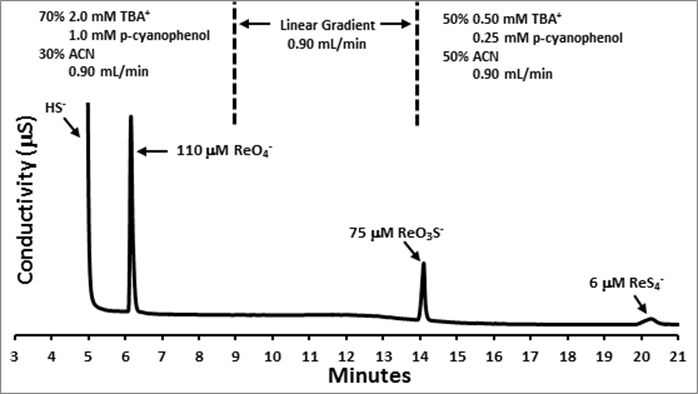
Figure 1 displays a representative chromatogram for the separation of HS-, ReO4-, ReO3S-, and ReS4- using RP-IPC with suppressed conductivity detection; eluent conditions throughout the elution are given above the chromatogram. A gradient elution was employed to promote separation within ~20 minutes.
Figure 1: Chromatogram demonstrating the separation of ReO4-, ReO3S-, and ReS4-. TBA+ and ACN refer to tetrabutylammonium cation and acetonitrile. Peak shapes are improved by adding p-cyanophenol.
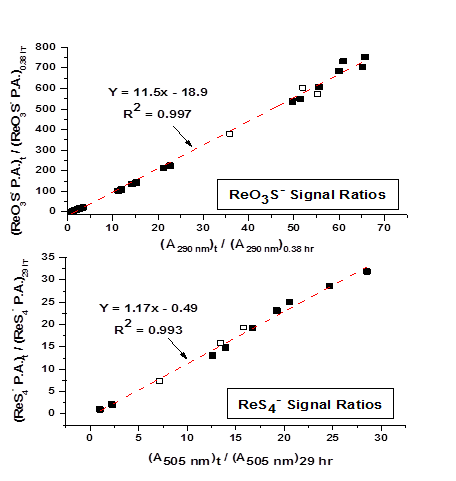
Unfortunately salts of ReO3S- and ReS4- are unavailable synthetically or commercially. To quantify these anions, RP-IPC peak areas had to be calibrated initially using optical constants. To accommodate this requirement, a test solution was prepared initially containing 2 mM ReO4-, 50 mM SS2-, and 50 mM NH4CH3CO2 at pH = 6.7. At various times, samples were withdrawn and immediately analyzed using UV-vis and RP-IPC. Figure 2 shows a plot of signal ratios from RP-IPC peak areas and UV-vis absorbances. The plot demonstrates that RP-IPC peak areas linearly correlate with UV-vis absorbances for ReO3S- and ReS4-.
Figure 2: Ratio of peak areas at a given time over the peak area at an initial time from RP-IPC vs. UV-vis absorbance ratios for ReO3S- and ReS4- for a solution initially containing 2 mM ReO4-, 50 mM SS2-, and 50 mM NH4CH3CO2 at pH = 6.7. Closed symbols represent samples with constant headspace. Open symbols represent samples experiencing elevated headspace for ~24 hours.
Figure 3 shows the reaction progress for the test solution described in Figure 2. ReO4-, ReO3S-, and ReS4- were quantified using RP-IPC peak areas. Dashed lines are spline or linear fits to the closed symbols which represent test samples experiencing constant headspace. Open symbols represent test samples experiencing elevated headspace for ~24 hours. Note that the samples experiencing prolonged elevated headspace in Fig. 3 show systematic deviations from the fitted trends. However, these deviations are not apparent in the calibration data shown in Fig. 2; RP-IPC and UV-vis quantified similar trends for these test samples. Reconnaissance experiments indicate that sulfidic solutions with elevated headspace may lose up to ~20% SS2- within ~24 hours. The loss of sulfide in the test samples points to hydrolysis of ReO3S- and ReS4- to ReO4- as the source of the systematic deviations. From a quantitative perspective, these results indicate that dilution of ReO3S- and ReS4- should be avoided. ReO3S- and ReS4- appear to be unstable even at relatively high H2S(aq) (>1 mM).
Figure 3: Reaction progress for the test solution described in Fig. 2. ReO4-, ReO3S-, and ReS4- were quantified using RP-IPC peak areas.
Preliminary experiments were undertaken to determine whether Re-polysulfido anions readily form in polysulfidic solutions. Two solutions were prepared initially containing 1 mM ReO4-, 25 mM SS2-, and 50 mM NH4CH3CO2 at pH = 6.7 with and without added elemental sulfur. The solutions were sampled after 6.8 days of reaction and analyzed by RP-IPC. The resulting chromatograms are shown in Figure 4. Peaks appearing between ReO3S- and ReS4- in the top chromatogram for the S0-added solution are assumed to be Re-polysulfido anions. Chromatograms of control solutions of oxidized sulfur species indicate that Sn2-, S2O32-, SO32-, or SO42- elute within 10 minutes and cannot account for the unidentified peaks. These results augur well for characterizing Re-polysulfido formation reactions by RP-IPC.
Figure 4: Chromatograms of two solutions initially containing 1 mM ReO4-, 25 mM SS2-, and 50 mM NH4CH3CO2 at pH = 6.7 with (top) and without (bottom) added elemental sulfur after 6.8 days of reaction.
Student Participation and Presentations: A total of five undergraduates have participated in this project. Four students presented their results at the Spring 2013 ACS national meeting. The PI presented results to the Geochemistry division of ACS at the Fall 2013 national meeting.
Wagner C.T., Maloney M.M., Yanez P., and Vorlicek T.P. (2013) Re(VII) reduction in the presence of sorbed Fe(II): Plausible removal pathway in suboxic porewaters. Presented at the Chemical Education Undergraduate Poster Session Spring 2013 American Chemical Society National Meeting, New Orleans, LA.
Groskreutz L.M. and Vorlicek T.P. (2013) Quantification of thioperrhenates using reverse phase ion pair chromatography with suppressed conductivity detection. Presented to the Analytical Chemistry Division at the Spring 2013 American Chemical Society National Meeting, New Orleans, LA.
Chappaz A. and Vorlicek T.P. (2013) Importance of measuring thiometalates for understanding Mo and Re geochemistry in sulfidic waters. Presented to the Geochemistry Division at the Fall 2013 American Chemical Society National Meeting, Indianapolis, IN.
Future Work: The project intends to utilize the developed RP-IPC method to quantify equilibria and kinetic constants for the thioperrhenates and Re-polysulfido anions over a range of solution conditions. The project also seeks to survey a plausible thermodynamically-feasible but kinetically-sluggish suboxic pathway to Re deposition involving reduction to ReIVO2(s) by Fe(II). These experiments will involve quantifying ReO4- removal rates by RP-IPC in Fe(II)-containing solutions in the presence and absence of various semi-conductive mineral surfaces (e.g., hematite, aluminum oxides, clays) over a range of aqueous-phase conditions.
Copyright © 2014 American Chemical Society