58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1050801-DNI10: Novel CO2 Reduction Catalysts Driven by Visible Light
Feng Jiao, University of Delaware
In the second year of study, we investigated silver and copper based catalysts as potential candidates for photocatalytic CO2 reduction. In this report, we present our recent results on fabrication and structural characterization of nanoporous silver and copper catalysts. Some preliminary results for CO2 reduction are discussed.
The most important accomplishment we achieved in the past 12 months is the demonstration of the first example of nanoporous silver (np-Ag) catalyst, which is able to reduce CO2 electrochemically to CO in a highly efficient and selective way. Not only the porous structure creates an extremely large surface area for catalytic reaction, but also the highly curved internal surface generates a large number of highly active step sites for CO2 conversion, resulting in an exceptional activity that is over three orders magnitude higher than that of polycrystalline counterpart at a moderate overpotential of < 500 mV. More importantly, such a remarkable activity for CO2 electroreduction has been achieved with a CO faradaic efficiency of 92%.
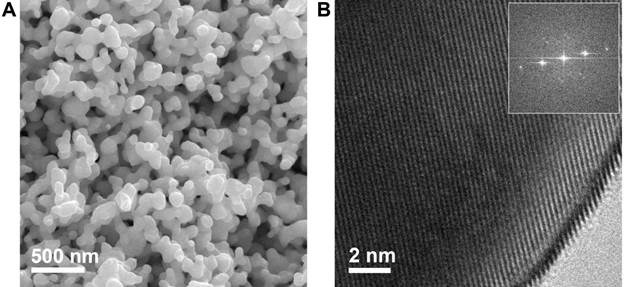
Figure 1. (A) Scanning electron micrograph of an np-Ag dealloyed in 5 wt% HCl for 15 minutes and further in 1 wt% HCl for 30 minutes. (B) Corresponding HRTEM with visible lattice fringes. Inset: The Fourier transform shows that the np-Ag is composed of an extended crystalline network.
The monolithic np-Ag catalyst were obtained by a two-step dealloy of Ag-Al precursors using aqueous HCl solutions. By selectively etching Al through dealloying, the remaining Ag atoms were reorganized to form a three-dimensional interconnected nanoporous structure. Such a dealloying process has been reported in other alloy systems as well. A typical scanning electron microscopy (SEM) image of the as-synthesized np-Ag catalyst is shown in Fig. 1A. The ligament size of np-Ag is approximately 50 ¨C 200 nm, while the size of the pores extends to a few hundred nanometers. High resolution transmission electron micrograph (HRTEM) exhibits uniform lattice fringes (Fig. 1B). Both techniques suggest that the resulting np-Ag is highly crystalline (single-crystal like).
CO2 reduction activity was measured using constant potential electrolysis experiments in an aqueous KHCO3 electrolyte (0.5 M). Testing was performed in an air-tight electrochemical cell with three electrodes and two compartments separated by an anion exchange membrane with potassium bicarbonate electrolyte in each chamber. Gas phase products from the headspace of the electrochemical cell were measured using a gas chromatography (GC) every 30 minutes. In addition, liquid phase products were measured using 1H NMR. Primarily carbon monoxide (CO) and hydrogen (H2) were detected using the GC, however trace amounts of formate (HCOO-) were detected using 1H NMR at potentials more negative than -0.60 V (vs. RHE).
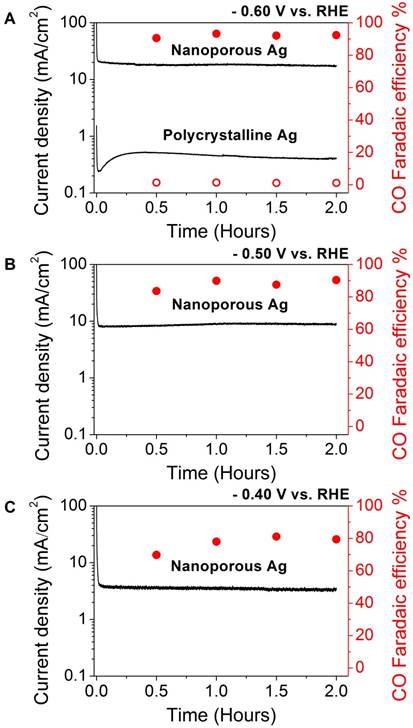
Figure 2. CO2 reduction activity of nanoporous silver and polycrystalline silver at (A) -0.60 V, and nanoporous silver at (B) -0.50 V, and (C) -0.40 V vs. RHE. Total current density versus time on (left axis) and CO faradaic efficiency vs. time (right axis).
The catalytic CO2 reduction results collected at -0.60 V (vs. RHE) are presented in Fig. 2A. The geometric current density is calculated based on electrode area. The operating voltage corresponds to an overpotential of 490 mV since the CO2/CO equilibrium potential is at -0.11 V vs. RHE. At this moderate overpotential, np-Ag electrode exhibited a long term stable current of ~18 mA/cm2 during the 2 hr electrolytic CO2 reduction. Faradaic efficiency for CO is maintained at ~92% throughout the whole electrocatalytic process, further confirming that the np-Ag catalyst is stable under electrocatalytic conditions once the surface oxide layer was removed electrochemically. In a sharp contrast, polycrystalline Ag exhibits a very low current density of 470 µA/cm2 with a poor CO Faradaic efficiency of ~1.1% at the same potential.
The performances of np-Ag at more positive potentials (-0.50 V and -0.40 V vs. RHE, corresponding to overpotentials of 390 and 290 mV, respectively) can be seen in Figs. 2B and C. With lower overpotentials, smaller currents were observed as expected, while CO faradaic efficiencies also decreased as potential decreased. At -0.50 V vs. RHE a stable current of ~9.0 mA/cm2 was observed with a CO efficiency of ~90%, whereas at -0.40 V vs. RHE a current of ~3.3 mA/cm2 was reached with a CO efficiency of ~79%. The decrease in CO faradaic corresponds to an increase in the relative rate of hydrogen evolution. This is most likely from the fact that the overpotential is relatively low to drive CO2 reduction quickly enough to compete with hydrogen evolution reaction, which only requires a small overpotential to occur.
To elucidate the origin of the high activity of np-Ag, we performed electrochemical surface area measurement and Tafel analysis. The electrochemically active surface area of np-Ag catalyst was measured by forming an oxide monolayer on the surface electrochemically. The np-Ag shows an electrochemical surface area (normalized to electrode area) 150°Á larger than that of polycrystalline Ag. Because the difference of current densities obtained at -0.60 V (vs. RHE) between np-Ag and polycrystalline Ag is 3000°Á, there is another 20 times difference that cannot be simply explained as surface area effect. We speculate that the intrinsic activity of catalytic sites on the nanoporous surface is much higher than those on a flat surface, resulting in 20 times difference in activities.
Figure 3. CO partial current density vs. overpotential (¦Ç) for nanoporous and polycrystalline silver.
To verify our speculation, Tafel analysis was performed to further determine the intrinsic activity of np-Ag for CO2 electrocatalytic reduction to CO (Fig. 3). Np-Ag was measured to have a Tafel slope of 58 mV/dec compared to 134 mV/dec for its polycrystalline counterpart. The dramatic decrease in Tafel slope for np-Ag indicates that in addition to 150 times larger electrochemical surface area np-Ag is also intrinsically better than polycrystalline Ag for CO2 reduction. The intrinsic activity accounts for about 20 times more CO than its polycrystalline counterpart at moderate overpotentials. With both high intrinsic activity and extremely large surface area, np-Ag simply represents the best reported electrocatalyst for CO2 electroreduction to CO selectively.
Copyright © 2014 American Chemical Society