58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND1051766-ND10: Synthesis and Characterization of Carbon Nanotube-Silicon Core-Shell Nanowire Array as Anode Material for Lithium-Ion Battery Application
Wenzhi Li, Florida International University
This New Directions grant enabled my group to initiate and establish a research program in advanced materials for energy applications. Student and postdoc researcher were educated and trained in energy science. The results obtained from this grant have been used in developing grant proposals submitted to the National Science Foundation to continue this research.
In the original proposal, Si was proposed to coat vertically aligned carbon nanotubes (CNTs) to form CNT-Si core-shell nanostructures. However, during the research, it was found that SnO2, another promising anode material for lithium ion battery application, can be uniformly synthesized on CNTs with a simple method at low cost while the formation of Si layer on CNTs presented an unexpected hurdle. Therefore, this progress report will be focused on the synthesis and characterization as well as the in-situ lithiation process of the CNT-SnO2 core-shell structures.
SnO2 based materials are suitable candidates for the future generation of anode materials for Li-ion batteries due to their high theoretical Li-ion storage capacity and low potential of Li-ion intercalation [1]. Despite having the maximum theoretical charge storage capacity (781 mAhg-1) over twice as much as the carbon anodes (372 mAhg-1), SnO2 based materials have to overcome the high volume change occurring during the charging and discharging processes. In order to improve the stability and reversible cyclic performance of the electrodes, SnO2 could be incorporated with CNTs to absorb the large stress developed during the alloying and dealloying processes. The high conductivity of CNTs could offer a quick pathway for electrons while the alignment of the CNT-SnO2 array will maximize the interface between the anode and the electrolyte. The in-situ synthesis of CNTs on conductive substrates and fabrication of CNT-SnO2 core-shell structures provides a direct platform for testing novel anode material for Li-ion battery.
Vertically aligned CNT array was synthesized on pristine stainless steel (SS) substrate, chemically etched SS substrate, and Ni/Cr layer coated SS substrate via plasma enhanced chemical vapor deposition (PECVD) method [2]. The effect of chemical etching on the surface morphology of the SS substrates and the growth of the CNTs were investigated. SS substrate chemically etched for 40 min resulted in CNTs with uniform diameter (95 nm) and length (3.4mm) (see Fig. 1). The experimental results indicate that a chemical etching to the SS substrate will expose the intrinsic Fe, Co, and Ni which can act as catalysts for the growth of CNTs. Hence, an additional catalyst would not be required for the synthesis of CNTs on SS [3].
Figure 1. SEM image of CNTs grown on SS substrates chemically etched in a mixture of H2SO4: H2O2: H2O (4:1:15, volume ratio) for 40 min. The CNT synthesis was carried at 600 °C for 10 min with C2H2 as precursor in PECVD.
Vertically aligned CNTs were coated with uniform layer of SnO2 through a simple hydrolysis route [4]. The as-synthesized CNTs was treated using HNO3 acid bath to introduce hydroxyl or carboxylic acid groups to the surface of CNTs to create a suitable hydrophilic environment necessary for the uniform coating of SnO2 nanoparticles on the CNTs [5]. As desired, the CNT arrays retained their structural integrity after the HNO3 treatment and the subsequent SnO2 coating procedure in a harsh chemical environment, in other words, the SnO2 coated CNTs are still vertically aligned, well-separated from each other, and strongly connected to the SS substrate, as shown in Figure 2. This remarkable stability indicates the robustness of CNT arrays grown on SS substrate and shows the possibility of using the CNT-SnO2 as anode materials for Li-ion batteries.
Figure 2. SEM image of SnO2 coated CNTs showing the retention of the alignment after SnO2 coating.
TEM investigation reveals that all CNTs have been fully coated with SnO2 particles to form core-shell CNT-SnO2 structures (Figure 3). It was reported that defect sites on the graphitic edges of CNTs is favorable for the formation of SnO2 nanoparticles on the CNT surface [5]. In our experiment, the CNTs synthesized by PECVD contain large amount of defects as a result of fast growth rate under the plasma. The high density of defects on the surface of CNTs leads to the formation of a layer of SnO2 nanoparticle on the entire surface of CNTs.
Fig. 3. TEM image of CNTs-SnO2 core-shell structures showing clearly the SnO2 coating layer on the CNT surface.
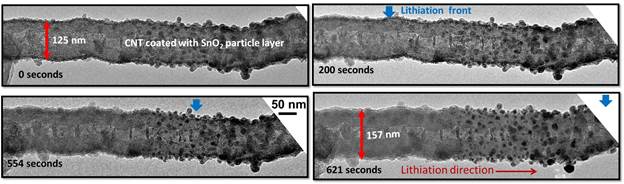
We have constructed a nanoscale electrochemical device using a single CNT-SnO2 nanowire as an anode inside a high-resolution transmission electron microscope to visualize the real-time electrochemical reaction-induced microstructural changes. The preliminary results show that the CNT-SnO2 lithiation and delithiation result in a significant morphology and structure change. The pristine SnO2 is tetragonal phase, and will change into hexagonal LixSn plus cubic Li2O upon lithaition. The spacing of (0002) plane of CNT increases after lithaition, indicating the Li intercalation (Figure 4). Upon cycles, the diameter of CNT-SnO2 increases, which suggests the growth of SEI layer. The result shows that small SnO2 particles on CNT are easily lithiated than large particles. Further experiments are ongoing to further understand the lithiation mechanism and charge capacity.
Figure 4. Volume expansion of a CNT-SiO2 due to the lithiation. The size of the CNT and SnO2 particles expanded after lithiation implying the lithiation of both the CNT core and the SnO2 nanoparticles.
1. Wang et al., Coating of multi-walled carbon nanotube with SnO2 films of controlled thickness and its application for Li-ion battery. J Power Sources 2008; 184(2):432-6.
2. Neupane et al., Synthesis and field emission properties of vertically aligned carbon nanotube arrays on copper. Carbon 2012; 50(7):2641-50.
3. Neupane et al., Synthesis and enhanced electron field emission of vertically aligned carbon nanotubes on stainless steel substrate, Journal of Nanoscience Letters, 2013. http://www.cognizure.com/jnl.aspx?b=1
4. Han et al., Coating single-walled carbon nanotubes with tin oxide. Nano Lett. 2003; 3(5):681-3.
5. Fang et al., Synthesis of tin (II or IV) oxide coated multiwall carbon nanotubes with controlled morphology. J. Phys. Chem. C 2008; 112(15):5790-4.
Copyright © 2014 American Chemical Society