58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR650670-UR6: Petroleomics: Elucidation of Chemical Composition and Functionality of Compounds Resistant to Asphaltene Inhibitors
Geoffrey C. Klein, PhD, Christopher Newport University
Introduction
This ACS-PRF grant supports laboratory work on the characterization of crude oils and their asphaltene fractions for the determination of those compounds resistant to two types of asphaltenes inhibitors. The three specific aims of this study are to fully characterize (elemental formulae and functional group composition) the crude oil and asphaltene fraction of a number of different crude oils, determine the effect amphiphiles have on the inhibition of asphaltenes precipitation and use model compound fragmentation studies to investigate functional group composition. The use of mass spectrometric techniques and Infrared spectroscopy will provide detailed elemental and functional group composition for the comparison of the asphaltenes compounds throughout the titration experiment.
Research Progress
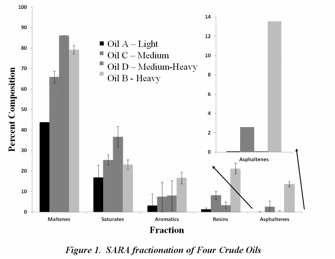
In the second year of the grant, we have refocused on the bulk analysis of the crude oils and their fractions. After Fourier Transform Ion Cyclotron Resonance (FT-ICR) mass spectrometric data was collected on the bulk oil and asphaltene fractions this past year, it was found that our extraction technique not only caused precipitation of asphaltenes, but also the co-precipitation of maltenes. This past year, the research lab has worked on the extraction of asphaltenes via the IP-143 method and the characterization of all SARA fractions (saturates, aromatics, resins, and ashaltenes). Figure 1 represents the four crude oils under investigation and the percent composition of each fraction. As one would predict, those oils that are medium-heavy have a greater percentage of aromatics, resins and asphaltenes. The low percentage of asphaltenes in Oil D (Medium – Heavy) is due to filtration with a 10 μm filter prior to analysis.
Aim 1
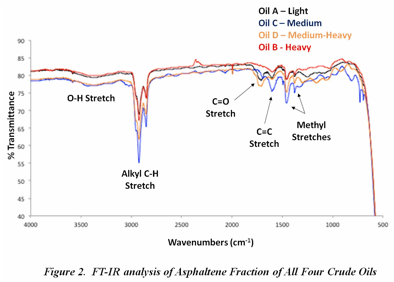
The fractions then underwent FT-IR analysis for the determination of functional group composition. All fractions had similar functional group composition, data not shown. Figure 2 illustrates the functional group characterization for the asphaltene fractions for all four oils. All four asphaltene fractions contain a broad band corresponding to O-H bonds at 3358 cm-1 and sharp peaks for C-H axial stretching at 2923 and 2852 cm-1. The stretch at 1602 cm-1 illustrates the presence of C=C bonds associated with aromatic structures. The asphaltene fractions of all four oils have relatively the same functional group composition. Further analysis by Principal Component Analysis will shed light on any differences that might be present.
The use of elemental analysis was used to ascertain the pure extraction of asphaltenes. Asphaltenes are known to have a H:C ratios less than 1.20, indicative of the high aromaticity of the fraction. The research group successfully employed the extraction technique. Table 1 indicates the H:C ratios for the four crude oils and their fractions. The low H:C ratios for Oils A, C, and B indicate that the asphaltene fractions have been collected, without the co-precipitation of the maltenes. The low composition of asphaltenes in Oil D makes it difficult to separate the asphaltenes without co-precipitation. In addition, the maltene fraction of all four oils have a higher H:C ratio, indicating the presence of highly saturated constituents. The high percentage of aromatic and resin components in Oil B, seen in Figure 1, corroborates the presence of the lowest H:C ratio for the maltenes. Further analysis of the elemental composition, such as nitrogen and oxygen composition, could shed light on the types of compounds present in each of the fractions.
Table 1. H:C Ratio ± St. Dev. of the fractions of each crude oil.
| |||||
Oil Sample
| Asphaltenes
| Maltenes
| Saturates
| Aromatics
| Resins
|
A (Light)
| 1.241 ± 0.004 | 1.808 ± 0.003 | 1.89 ± 0.02 | 1.12 ± 0.01 | 1.21 ± 0.007 |
C (Medium)
| 1.00 ± 0.02 | 1.66 ± 0.04 | 1.88 ± 0.02 | 1.31 ± 0.04 | 1.29 ± 0.01 |
D (Medium-Heavy)
| 1.44 ± 0.01 | 1.677 ± 0.002 | 1.68 ± 0.009 | 1.34 ± 0.06 | 1.35 ± 0.01 |
B (Heavy)
| 1.17 ± 0.07 | 1.52 ± 0.06 | 1.72 ± 0.006 | 1.31 ± 0.01 | 1.34 ± 0.003 |
Numerous papers were published this past year indicating the difficulty associated with the analysis of the asphaltene fraction via ESI FT-ICR MS. The ability of this fraction to form nanoaggregates makes it difficult to analyze this fraction via mass spectrometry. While research into the analysis of the asphaltene fraction via FT-ICR MS will continue, we have shifted our focus to the ability of the crude oil, or maltene fraction, to solubilize the asphaltene fraction. There have been few studies that have investigated the composition of the maltene fraction as the factor that affects its ability to stabilize the asphaltene fraction during refining processes. The Hildebrand Solubility Parameter is one means to semi-quantitatively define the ability of the oil to stabilize the asphaltene fraction. The solubility parameters of the oils and their asphaltenes have been determined, data not shown.
Aim 2
Progression on Aim 2 of the grant has begun. The inhibitors (both ionic and nonionic) have been purchased and we are currently investigating the best experimental conditions to conduct the study, including inhibitor concentration, crude oil conditions, and spectroscopic detection of asphaltene flocculation point. Both inhibitors will be applied to all four crude oils and changes in the Hildebrand Solubility Parameters will be determined. Flocculated asphaltenes will be collected and analyzed via elemental analysis and FT-IR spectroscopy.
Aim 3
Progression towards the completion of Aim 3 has stopped due to the inability of FT-ICR MS to analyze the complete asphaltene fraction. Future work will be dependent of the development of an experimental means to prevent the formation of asphaltene nanoaggregates during the ionization process of the analysis.
Conclusion
Work towards the completion of Aim 1 continues and should be completed in the coming months. Multiple techniques have been applied for the determination of bulk oil properties and detailed elemental composition analysis of the different fractions of all four crude oils. Collaboration with the NHMFL ICR team on the use of FT-ICR MS analysis continues to provide expose CNU undergraduate students to highly advanced analytical techniques. The PRF grant has provided summer support for two undergraduate students during this funding period. In addition, the principal investigator and the undergraduate students gave three presentations at regional and national conferences.
Copyright © 2014 American Chemical Society