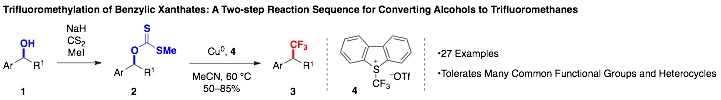
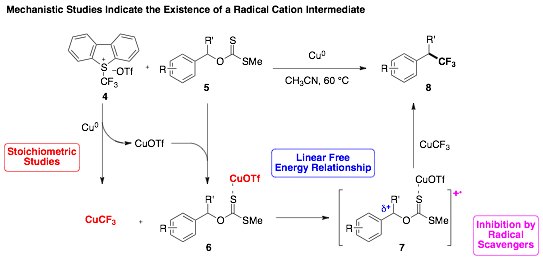
58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI152073-DNI1: Development of Novel, Stable and User-Friendly Trifluoromethylation Reagents
Ryan A. Altman, PhD, University of Kansas
Copyright © 2014 American Chemical Society