58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR351747-UR3: Hydrogen Production via Homogeneous Catalysis: Investigations of the Water-Gas-Shift Reaction
Jason S. D'Acchioli, PhD, University of Wisconsin (Stevens Point)
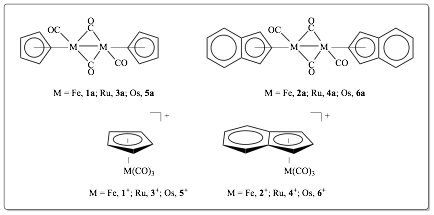
1. Introduction. The synthesis and characterization of a series of Group 8 organometallic complexes (Figure 1) has been undertaken in order to probe their involvement in the so-called water-gas-shift (WGS) reaction (Figure 2).
Figure 1. Target complexes under investigation.
Figure 2. The water-gas-shift reaction.
2. Progress.
2.1 Syntheses. Complex 1a is commercially available through the Strem chemical company. Synthesis of 1+ from 1a was achieved at a 80% yield. Yields of 3a and 3+ were 47% and 4%, respectively. Complexes 2a and 2+ were observed by IR spectroscopy, but yields were too poor for isolation. Our group is attempting to optimize those syntheses. Complexes 2a and 4+ are easily made by our research group.1
Synthesis of the osmium complexes 5+ and 6+ may not require the initially planned synthesis of 5a and 6a. The synthesis of viable precursors for 5+ and 6+ will, however, require fairly stringent safety precautions since they utilize high-pressure glassware.2 We will attempt syntheses of these complexes throughout the upcoming grant cycle.
2.2 Computational studies. A literature investigation into the best choice of level of theory was undertaken. Based on the work of Frenking and others, we have determined that geometry optimizations and frequency calculations for structures will be at the B3LYP/6-31G(d,p) level of theory.3-5 To determine the best possible energies for structures involved in any reaction steps we will calculate energies at the CCSD(T)/6-31++G(d,p) level of theory. Zero-point energy corrections from the aforementioned B3LYP/6-31G(d,p) level of theory will be applied to the CCSD(T) results.
2.3 Mass spectrometric determination of water-gas-shift activity. There is literature precedence for using mass-spectrometric detection of CO2 as a handle for detecting the water-gas-shift reaction (Figure 2).6 Direct detection of hydrogen gas would be more ideal, so we initially purchased a more selective (for lower molecular weights) low-gauss magnet for our GC 6890N / MS 5973 magnet. After initial attempts with this magnet we discovered our system would need a low-temperature setup to be truly effective. We returned the magnet, and returned to using CO2 as our handle. Initial evidence shows 3+ being water-gas-shift active, but not 1+. Further investigations are underway.
3. Impact on PI. The acquisition of the PRF grant has had a significant impact on the PI's career. First, the grant favorably impacted the PI's tenure decision and promotion to associate professor in the spring of 2012. Second, the PI was honored in the spring of 2013 with an institutional "Excellence in Teaching" award, given in recognition of the PI's continued use of chemical research as a pedagogical tool at the undergraduate level. The award is given annually based on the vetting, and selection, of 3-4 campus-wide recipients by a university committee.
4. Impact on Undergraduates. The students involved in this project have significantly expanded not only their chemical knowledge, but also their ability to communicate results to scientists and non-scientists. Students presented results at an undergraduate research symposium held at the state capitol in the spring of 2012. The purpose of the annual event is to help underscore the importance of funding undergraduate research experiences to state lawmakers. Students also presented results at a university wide research symposium during the 2012 and 2013 spring semesters. Students involved in this project have indicated a desire to pursue chemical research at the graduate level.
5. References.
1. Badger, R. C.; D'Acchioli, J. S.; Gamoke, B. C.; Kim, S. B.; Oudenhoven, T. A.; Sweigart, D. A.; Tanke, R. S. Organometallics. 2009, 28, 418-424.
2. Allcock, H. R., Inorg. Synth. John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1989; Vol. 25, p 187-192.
3. Sola, M.; Frenking, G. Organometallics. 1999, 18 2801-2812.
4. Vuilleumier, R.; Rozanska, X. Inorg. Chem. 2008, 47, 8635-8640.
5. Zhang, F.; Zhao, L.; Xu, C.; Chen, Y. Inorg. Chem. 2010, 49, 3278-3281.
6. Badger, R. C.; D'Acchioli, J. S.; Oudenhoven, T. A.; Walder, B. J. Organometallics. 2010, 29, 1061-1063.
Copyright © 2014 American Chemical Society